This course covers pathophysiologic concepts and nursing interventions for patients with heart and coronary vessel disease and disorders. The pathology of physiologic function is detailed for coronary heart disease (CHD), angina pectoris, myocardial infarction (MI), congestive heart failure (CHF), dysrhythmias, inflammatory processes, and valvular heart disease. Associated pathophysiology is covered in each disease presentation and includes diagnostic examinations and needed laboratory tests and procedures relevant for that health problem. Nursing assessment strategies, care planning, interventional management, and patient teaching are addressed. This course is designed to broaden the nurse's understanding of pathophysiology by exploring causes, alterations and physiology adaptations, manifestations, and resolution of disease states. Pathophysiologic symptoms and signs are described in relation to the patient's clinical presentation, allowing the nurse to monitor physical changes and relate them directly to the illness process. Appropriate diagnostic tests and treatments for each problem are included along with the nurse's responsibilities for patient teaching about these experiences. Information about disease progression, remission, and resolution may also be found in the case studies included in the course.
- INTRODUCTION
- THE HEART: STRUCTURAL AND FUNCTIONAL INTER-RELATIONSHIPS
- REGULATORY FUNCTIONS OF THE CARDIOVASCULAR SYSTEM
- PATHOPHYSIOLOGIC INFLUENCES AND EFFECTS
- RELATED SYSTEM INFLUENCES AND EFFECTS
- PSYCHOSOCIAL/LIFESTYLE INFLUENCES AND EFFECTS
- NURSING ASSESSMENT: ESTABLISHING THE DATA BASE
- CARDIOVASCULAR DISEASES
- CARDIAC SURGERY
- CASE STUDIES
- CONCLUSION
- Works Cited
- Evidence-Based Practice Recommendations Citations
This course is designed for nurses working in both critical care and coronary care units and those on any medical-surgical unit in which patients with multiple organ system problems are found.
As health care becomes more complex, it is essential that the theoretical concepts of the basis of illness (pathophysiology) be well understood. The purpose of this course is to reinforce the scientific rationales for the interventions nurses perform and the decisions nurses make as patients move through the ever-changing struggle with their illness.
Upon completion of this course, you should be able to:
- Describe the key structures and functional inter-relationships in the cardiovascular system.
- Identify the regulatory functions of the cardiovascular system.
- Discuss the pathophysiologic and environmental influences and effects on the cardiovascular system.
- Outline the role of subjective data in completing a full nursing assessment of the cardiovascular system.
- Describe objective data compiled during a nursing assessment of the cardiovascular system.
- Identify elevated enzyme changes in myocardial infarction and the significance of the elevation.
- Analyze electrocardiographic tracing appearance characteristics in patients with cardiovascular disease.
- Evaluate cardiac catheterization results in the nursing diagnosis of cardiovascular disease.
- Identify other diagnostic tests used in the identification and classification of cardiovascular diseases.
- Outline the nursing diagnosis and management of angina pectoris and myocardial infarction.
- Review signs and symptoms of congestive heart failure and related nursing actions.
- Describe the common causes, appearances, and treatment of dysrhythmias.
- Identify pathologic causes and manifestations of inflammatory diseases of the heart.
- Discuss the pathophysiology and clinical manifestations of valvular heart disease processes.
- Discuss the concepts and information the nurse should provide for the patient during the health teaching and discharge planning process after cardiac surgery.
Mary Franks, MSN, APRN, FNP-C, is a board-certified Family Nurse Practitioner and NetCE Nurse Planner. She works as a Nurse Division Planner for NetCE and a per diem nurse practitioner in urgent care in Central Illinois. Mary graduated with her Associate’s degree in nursing from Carl Sandburg College, her BSN from OSF Saint Francis Medical Center College of Nursing in 2013, and her MSN with a focus on nursing education from Chamberlain University in 2017. She received a second master's degree in nursing as a Family Nurse Practitioner from Chamberlain University in 2019. She is an adjunct faculty member for a local university in Central Illinois in the MSN FNP program. Her previous nursing experience includes emergency/trauma nursing, critical care nursing, surgery, pediatrics, and urgent care. As a nurse practitioner, she has practiced as a primary care provider for long-term care facilities and school-based health services. She enjoys caring for minor illnesses and injuries, prevention of disease processes, health, and wellness. In her spare time, she stays busy with her two children and husband, coaching baseball, staying active with her own personal fitness journey, and cooking. She is a member of the American Association of Nurse Practitioners and the Illinois Society of Advanced Practice Nursing, for which she is a member of the bylaws committee.
Contributing faculty, Mary Franks, MSN, APRN, FNP-C, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Margo A. Halm, RN, PhD, NEA-BC, FAAN
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#38833: Pathophysiology: The Cardiovascular System
Pathophysiology is the study of disordered and altered functions affecting the body's dynamic homeostasis and the concepts of disease development and progression. This course covers pathophysiologic concepts and nursing interventions for patients with heart and coronary vessel disease and disorders. The pathology of physiologic function is detailed for coronary heart disease (CHD), angina pectoris, myocardial infarction (MI), congestive heart failure (CHF), dysrhythmias, inflammatory processes, and valvular heart disease. Associated pathophysiology is covered in each disease presentation and includes diagnostic examinations and needed laboratory tests and procedures relevant for that health problem. Nursing assessment strategies, care planning, interventional management, and patient teaching are addressed. This course is designed to broaden the nurse's understanding of pathophysiology by exploring causes, alterations and physiology adaptations, manifestations, and resolution of disease states. Pathophysiologic symptoms and signs are described in relation to the patient's clinical presentation, allowing the nurse to monitor physical changes and relate them directly to the illness process. Appropriate diagnostic tests and treatments for each problem are included along with the nurse's responsibilities for patient teaching about these experiences. Information about disease progression, remission, and resolution may also be found in the case studies included in this course.
The heart, which functions as two pumps, contracts and generates pressure to push the blood through the entire vascular system and back. The right side of the heart generates the pressure required to pump blood through the capillaries of the lungs, where exchange of gases—primarily oxygen and carbon dioxide—occurs, and to the left side of the heart. The left side of the heart generates pressure to push the blood throughout the systemic circulation (where nutrients, waste products, and a variety of other chemical substances are exchanged between the capillaries and interstitial spaces or the cellular internal environments) and back to the right side of the heart, where the process is repeated [1].
The vascular system consists of arteries, arterioles, capillaries, venules, and veins, which provide the conduit for blood flow. The arteries promote blood flow throughout the system and divert it to the capillaries. There the blood provides nutrients or body defense substances when and where needed and removes waste byproducts from the cellular environment. The arteries also route the blood to areas where nutrients, body defense substances, and chemicals are picked up to be distributed elsewhere in the body. They transport the blood to areas where waste products either can be reprocessed or removed, such as the liver and kidneys. The arteries contribute to body temperature regulation by diverting blood to the skin, where heat can be released, or diverting blood away from the skin to conserve body heat. An artery less than 0.5 mm in diameter is an arteriole. Small arteries and arterioles are called resistance vessels because they regulate the volume and pressure in the arterial system and blood flow into the capillary bed via dilation or constriction [2].
Venules, which receive their blood from the capillaries, converge to form veins of progressively larger diameters. The veins carry the blood back to the right side of the heart (or to the left side from the lungs). They also serve as reservoirs for blood when the body's needs are reduced. The capillaries serve not only as conduits for blood flow, but also as sites for the exchanges between the interstitial spaces and the blood.
The lymphatic system acts as an accessory circulation for the fluids and particles that leave the capillaries and do not return by the same route. The lymphatics pick up excess fluid and larger particles (e.g., proteins) and return them to the circulatory system. The lymphatics also protect the body against infection [2].
The blood consists of erythrocytes (red cells), leukocytes (white cells), thrombocytes (platelets), clotting factors, water, oxygen, carbon dioxide, electrolytes, vitamins, minerals, sugars, proteins, fats, hormones, and other chemical substances, as well as waste products. The quality and quantity of the blood determine whether the circulatory system can fulfill its purpose.
In studying cardiovascular dynamics, the nurse should understand and be able to apply some laws of physics. Perhaps the most important is Poiseuille's law, which holds that the flow rate of a fluid through a tube is proportional to pressure differences and the diameter of the tube in relation to the length of the tube and the viscosity of the fluid. In the cardiovascular system, the heart generates the pressure; the diameter and length of the tubes are determined by the blood vessels; and the blood has a certain viscosity. To visualize the application of this law, imagine giving an injection. The more pressure applied to the plunger, the faster the fluid flows. The smaller the diameter and the longer the needle, the greater the pressure needed to make the fluid flow; and the more viscous the fluid, the greater the pressure needed to make the fluid flow. Factors that hinder flow involve resistance, which in this case is determined by the diameter and length of the tube as well as the viscosity of the fluid. In addition, the pressure depends on the amount of fluid in the container [3].
The cardiac output, or the amount of blood the heart pumps per minute, is the amount of blood ejected from the left ventricle into the aorta per minute. This is determined by the amount of blood the heart pumps with each stroke (stroke volume) and the number of strokes per minute (heart rate). The heart pumps approximately 70 mL of blood with each stroke at an average rate of 72 strokes per minute; this accounts for an average of more than 5,000 mL of blood per minute. The heart can increase this quantity of blood by about four times by increasing the heart rate, the amount of blood it pumps with each stroke, or both. The heart's ability to do this depends on its ability to time contractions, its ability to contract, and the amount of blood available to pump. These capabilities of the heart first will be described in an overall presentation of blood flow through the heart, contractility of the heart, electrical conduction in the heart, and coronary circulation in relation to anatomy and physiology. Factors outside the heart that influence these variables will be discussed later in relation to their influences on the whole system [2].
The heart, a four-chambered structure about the size of a fist, is located in the mediastinum of the chest just beneath the sternum. The upper chambers are called the atria, and the lower are the ventricles. The chambers are separated by valves. The valve separating the right chambers, with three cusps, is called the tricuspid valve. The valve separating the left chambers is called the bicuspid or mitral valve and has two cusps. Both are referred to as the atrioventricular (AV) valves. Two other valves in the heart open when blood leaves the ventricles: the pulmonic valve on the right and the aortic valve on the left. They are named for the arteries into which they open and are often referred to as the semilunar valves [2].
A key point to keep in mind while tracing blood flow is that fluids move from an area of higher pressure to one of lower pressure. Blood flows from the venous system (which averages about 5 mm Hg pressure) into the right atrium (which averages about 8 mm Hg pressure) via the vena cava and coronary sinus. While the right ventricle is in relaxation, or diastole, blood continues to flow through the tricuspid valve and into the right ventricle. When the right ventricle is 70% to 75% full, the right atrium contracts (causing a rise in pressure) and fills the right ventricle about 25% to 30% more. Shortly after the right atrium completes its contraction (right atrial systole), the right ventricle begins to contract (right ventricular systole) and generates enough pressure (about 22 mm Hg) to cause the tricuspid valve to close, the pulmonic valve to open, and blood to flow into the pulmonary artery. While the right ventricle is in systole, the right atrium is in diastole and collects blood from the venous system [2,3].
Simultaneously, similar activity is happening in the left side of the heart. Blood flows freely into the left atrium and through the mitral valve into the left ventricle during left ventricular diastole, as the blood pressure in both chambers is near 0 mm Hg. When the left ventricle is 70% to 75% full, the left atrium contracts to fill the left ventricle another 25% to 30%. Shortly after left atrial systole, the left ventricle begins to contract (left ventricular systole) and generates enough pressure (about 120 mm Hg) to cause the mitral valve to close, the aortic valve to open, and blood to flow into the aorta to be carried into the systemic circulation. As the ventricles relax (ventricular diastole), the pressure drops, and blood attempts to flow backward through the semilunar valves. Because these valves are shaped like cups, blood fills them up and closes the valves. This is important because the arteries that feed the heart muscle are located near the cusps of the aortic valve and fill during diastole [2,3].
The left ventricle generates a higher pressure than the right; this is logical, because the left side of the heart needs to pump the blood a greater distance. The left ventricle has a greater workload, needs more muscle mass, and therefore has a thicker muscular wall. If the arterial diastolic pressure rises, promoting more resistance to flow as it does in hypertension, the left ventricle must work harder [2].
Blood flows from the aorta throughout the systemic circulation and back to the heart via the right atrium because of a difference in blood pressures. In the normal young adult, the pressure in the aorta averages about 100 mm Hg, whereas the pressure in the right atrium is about 0 mm Hg. These values increase after middle age. This pressure difference produces a pressure gradient, and blood flows from the area of higher pressure to the area of lower pressure [3].
The AV valves close during systole but do not convex because of two important structures: the chordae tendineae and the papillary muscles. The chordae tendineae are white, fibrous chords that connect the valve leaflets to the papillary muscles. The papillary muscles arise from the walls of the ventricles and contract during ventricular systole to prevent the valves from bulging into the atria [2,3].
The heart has three layers: the endocardium, the myocardium, and the pericardium. The endocardium is the smooth layer of endothelial tissue that lines the inside of the heart and covers the valves. The myocardium is the thick layer composed of striated muscle fibers that are responsible for contraction and pumping of blood. The pericardium is the covering of the heart composed of serous and fibrous layers separated by the pericardial fluid-containing space. The visceral pericardium (epicardium) lines the outermost fibrous pericardium. The pericardial space contains about 5 to 20 mL of fluid and protects the heart from friction during contractions and relaxations [2,3].
Within its physiologic limits, the heart is able to pump all the blood returning to it without allowing it to back up into the veins. When the heart is in the contraction phase, this is measured by systolic blood pressure. Because the body tissues regulate their own blood flow according to their needs, the amount of blood returning to the heart varies from moment to moment, and the heart is able to accommodate these changes. The muscle fibers of the heart act similarly to skeletal muscle fibers when stretched. The more they are stretched within physiologic limits, the greater the force of contraction. The myocardial fibers stretch with increased amounts of returning blood during diastole and contract with greater force during systole (called the Frank-Starling law of the heart). Preload is the term that refers to the degree of stretch of myocardial fibers before contraction. Afterload is the term that refers to the tension the ventricles develop in systole to pump against pressure in the aortic valve and aorta and resistance in the systemic and pulmonary arterioles [4,5].
The cardiac muscle fibers are similar to skeletal muscle fibers in that they have actin and myosin protein filaments that slide along one another, producing contraction. They are different from skeletal muscle fibers in that the cardiac muscle cells are separated from one another by intercalated discs—the cardiac muscle cell membranes. The intercalated discs allow free diffusion from cell membrane to cell membrane; this diffusion plays an important part in the transmission of the electrical impulse from one cell to another, because it is the electrical change that signals muscular contraction. Another characteristic of the cardiac muscle cells is that essentially all of them can spontaneously contract and relax when isolated [4,5].
The heart has three types of muscle fibers: atrial, ventricular, and conducting. Although similar, the atrial and ventricular fibers are completely separated from one another. Their only connection is by way of specialized conducting fibers between the atria and ventricles. The conducting fibers differ from the atrial and ventricular fibers in that they have fewer contractile fibers and therefore have much less contractile ability. They especially are different in their ability to transmit electrical impulses much more rapidly than other kinds of muscle fibers. The point of maximal impulse is located near the apex of the heart [4,5].
When cardiac muscle cells are at rest, the inside of the cardiac cell membrane is electrically negative in relation to the outside. This electrical situation is referred to as the resting membrane potential. When the cell membrane is stimulated (and in some instances without stimulation), the cell pores (channels) open, making the cell membrane permeable to sodium. Sodium ions rush into the cell, and the cell membrane loses its electronegativity in a process referred to as depolarization. During the plateau phase, calcium ions defuse into the myofibrils and begin to catalyze the reaction that causes the actin and myosin filaments to slide over one another to effect contraction. Suddenly, the cell membrane becomes impermeable to sodium ions and permeable to potassium ions. Potassium ions rush out of the cell and promote the return to the resting membrane potential, or repolarization. After repolarization, calcium ions diffuse out of the myofibrils, causing muscular relaxation. This entire process of depolarization and repolarization is called the action potential [4,5].
This electrical activity in the heart can be visualized by putting electrical sensors on the surface of the skin and recording the activity by use of the electrocardiogram (ECG). Imagine the cells in a resting state as dominoes that are standing up, side by side, waiting to be knocked down. When a domino falls, it depolarizes; when it automatically gets up and is capable of falling down again, it is repolarized. These are special dominoes, because when one domino falls, it can knock down all the dominoes close to it—ahead, behind or to the side of it—and all of them at the same time. The influx of sodium causes the dominoes to fall and subsequently causes the next domino to fall. Realize that a domino can knock down any adjacent domino, but it knocks down those near the intercalated disc faster. Some pathways are faster, and some take longer. The fast pathways are through the specialized conductive tissue; conduction through muscle mass takes longer. The only way the atrial dominoes can knock down the ventricular dominoes is through the specialized conductive tissue between the atria and ventricles. The ECG detects the direction in which the dominoes (cells) are falling (depolarizing) and getting up (repolarizing) [4,5].
The fast conductive fibers important in understanding impulse transmission through the heart include the sinoatrial (SA) node, the AV node, the bundle of His, the right and left bundle branches, and the Purkinje fibers. Essentially, all the cells of the heart are believed capable of automaticity (spontaneous depolarization without external stimulation). The SA node, located in the right atrium medial to the junction of the right atrium and the superior vena cava, has the fastest intrinsic rate for spontaneous depolarization. The SA node normally spontaneously depolarizes between 70 and 80 times per minute. The fast conductive fibers in the area of the AV node, located just beneath the septal wall of the atria, have an intrinsic rate of about 40 to 60 spontaneous depolarizations per minute. The bundle of His is continuous with the AV node, the left bundle branch (which runs along the left interventricular septum), the right bundle branch (which runs along the right interventricular septum), and the terminal Purkinje network (which penetrates the heart muscle). The Purkinje fibers have an intrinsic rate of between 15 and 40 spontaneous depolarizations per minute [4,5].
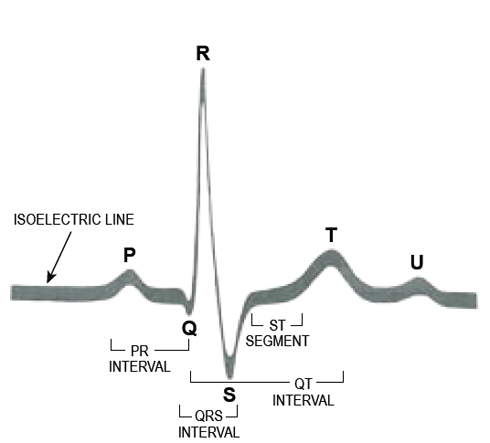
The SA node is called the "pacemaker of the heart," because its depolarization wave spreads through the heart and causes depolarization of the heart cells before any other cells spontaneously depolarize. Normally, the depolarization wave spreads rapidly through these extremely fast fibers and spreads less quickly through the muscle walls, signaling the muscle cells to contract. The electrical events of the ECG inscription are described as P, Q, R, S, and T waves (Figure 1). The SA node initiates the impulse, and each is spread through the atria into the AV node, where the impulse is delayed while the atria contract to fill the ventricles completely. This produces a P wave on the ECG, signifying transmission of the depolarization wave through the atria. (The wave is written upright, because the sensor is near the apex of the heart.) There is a period of no electrical activity (signified by a straight line on the ECG) while the ventricles are filling (called the PR interval). Suddenly, the wave spreads through the bundle of His and into the ventricular septum (from left to right), which causes the inscription of the Q wave (the first negative deflection). The depolarization wave spreads rapidly through the right and left ventricles. Because the left ventricle has many more cells, the electrical sensors draw an upward R wave. The last part of the ventricle to depolarize is the left upper wall of the left ventricle, which causes the sensor to draw a small downward deflection, called the S wave. Atrial repolarization cannot be seen on the normal ECG because it occurs during ventricular depolarization and is hidden in the QRS complex. Another straight line signifies no electrical activity. Shortly thereafter, a T wave forms, which the sensor records when the ventricular muscle cells repolarize. Because ventricular repolarization occurs in a different sequence than depolarization, the T wave is upright [6,7].
There are normal time limits for these events to occur. During the ECG recording, the paper marked with light and dark horizontal lines moves under the writing instrument at a standard rate. The distance of five dark lines (counting the origin would make six) equals 1 second. The distance between two dark lines equals 0.2 seconds. The time necessary for the depolarization wave to be conducted through the various parts of the heart is significant in evaluating abnormalities of the heartbeat [9].
The function of the coronary arteries is to provide blood to the myocardium. Like the other cells of the body, the heart needs blood flow to maintain cellular life and perform its work. It receives its blood supply from the left and right coronary arteries, which originate in the root of the aorta just above the left posterior and anterior cusps of the aortic valve. Blood flows into these arteries during ventricular diastole, when the aortic valve is closed by blood pushed backward by the recoil of the aorta at the beginning of ventricular diastole. The duration of ventricular diastole is important to ensure that the heart cells receive sufficient blood supply. Note that when the heart cells contract, the contraction exerts pressure against the coronary vessels and inhibits blood flow [2,3].
The left coronary artery primarily supplies the left atrium and the majority of the left ventricle. This artery branches into the left anterior descending artery and the circumflex artery. In most people, the left anterior descending artery and its branches supply the interventricular septum and surrounding myocardium. The circumflex artery and its branches supply the lateral wall and part of the posterior wall of the left ventricle. The right coronary artery supplies the right atrium, the right ventricle, and in most persons, the AV nodes and the inferior and posterior portions of the left ventricle.
Most cardiac veins empty into the coronary sinus, which drains directly into the right atrium. Some veins empty directly into all chambers of the heart [2,3].
As discussed, the blood vessels carry the blood from the heart, provide an area for exchange of substances between the blood and interstitial spaces, and return the blood back to the heart to be recirculated. Generally, the arteries and arterioles carry the blood to the capillaries (or sinusoids, which are larger and more irregular tubes such as those found in the endocrine glands, liver, bone marrow, and spleen). In the capillaries and sinusoids, exchange takes place, and the venules and veins carry the blood back to the heart for recirculation [4]. (Some exchange also takes place in the capillary ends of the venules.)
All blood vessels except the capillaries are innervated by sensory and vasomotor nerves and have three layers of tissue. The inner layer, lined with a smooth layer of endothelial cells supported by a layer of connective tissue, is called the tunica intima; the middle layer of collagen and elastin fibers is called the tunica media; and the outer layer of collagen and elastin fibers is called the tunica adventitia. The vessels have small nutrient vessels (vasa vasorum) that run into the tunica adventitia and the tunica media, and most have lymphatic vessels. The size of the blood vessels and the amount of muscular tissue vary according to the blood pressures within the vessels. The capillaries consist of a single layer of endothelial cells covered by a glycoprotein layer that merges into an adventitious layer of fine reticular tissue. The structure of the vessels is related to their function [4].
The arteries are sometimes referred to as distribution or conduction vessels because they provide continuous blood flow to the periphery. Their walls are thick and elastic, enabling them to stretch to accommodate greater amounts of blood under high pressure during ventricular systole. Because of their elasticity, the arteries recoil during ventricular diastole and maintain sufficient blood pressure to promote blood flow through the system. They branch like a tree, and the branches get smaller as they extend away from the heart. The arteries are also called the high-pressure blood vessels [4].
Not all arteries end in arterioles. Some anastomose (unite) with other arteries. There are more anastomoses as the arteries decrease in size and move away from the heart. Anastomoses are especially prominent around joints, where external pressure during movement momentarily interrupts circulation. The anastomosed artery provides the intermittent blood supply. When blood supply is compromised for a time from disease or injury, the anastomosed artery enlarges to provide collateral circulation. Sudden occlusion, however, may not permit sufficient time for the collateralization, causing the involved tissue to die [4,5].
Some arteries develop connecting vessels that join veins, resulting in arteriovenous shunts that divert the blood into the venous system rather than to the capillary. These shunts are especially common in the skin of the hands, feet, nose, and ears and function in temperature regulation. When the body temperature is low, the shunts open. Arteriovenous shunts are also common in the gastrointestinal tract. The connecting vessels close when absorption is taking place and open when it is not, shunting the blood to other areas.
Arterioles are short vessels (a few millimeters in length) that branch into terminal arterioles, which feed the capillaries. The arterioles, referred to as resistance vessels, have small diameters. The blood pressure drops from about 85 mm Hg when blood enters the arteriole to about 30 mm Hg at the beginning of the capillary [5].
In most tissue areas, each capillary is joined by an arteriole (sometimes called a metarteriole), which branches to serve other capillaries and ultimately joins a venule to form a capillary and closes to divert blood to the venule or other capillaries in the network. The periodic opening and closing (about 5 to 10 times per minute) of different sphincters ensures irrigation of various parts of the capillary network. The most important factor in the regulation of the opening and closing of these sphincters is the oxygen content in the tissues. Other substances that promote vasodilation are found in the area as well and may have some effect. When the oxygen concentration is low, the sphincters open more often and permit increased blood flow, resulting in more oxygen and nutrients to the area (autoregulation of blood supply) [4,5].
As noted, exchange takes place in the capillaries. The movement of substances both ways across the capillary membrane primarily occurs by diffusion, filtration, and osmosis. Primary factors that control the movement of fluid into the interstitial space include hydrostatic pressure (blood pressure), which tends to push fluid into the interstitial space, and colloidal osmotic (oncotic) pressure (primarily related to albumin and proteins), which tends to pull fluid in. The net effect is that fluid moves into the interstitial space at the arterial end, where hydrostatic pressure is about 30 mm Hg, and fluid moves back in at the venous side, where hydrostatic pressure is about 10 mm Hg (providing colloidal osmotic pressure is normal). Not all the fluid that leaves the capillaries returns to them. Some fluid returns to the venous circulation by way of the lymphatics, while some protein substances and other larger particles leak out and are also returned by the lymphatics [4,5].
The venules collect the blood from the capillaries and small arterioles. There may be some fluid exchange in the venules, and they also serve as one of the routes for the white blood cells' migration into and out of the tissues.
The veins are low-pressure vessels with thin walls and less muscle in the tunica media than arteries. They serve as conduits to return blood to the heart. Veins are referred to as capacitance or reservoir blood vessels because they hold more than 60% of the blood. With the exception of the largest and smallest veins, most have valves that prevent blood from moving backward. As the body muscles contract, they exert external pressure against the veins, which promotes the forward flow of blood. When the venous pressure falls, the valves prevent back flow until the muscles help move blood forward again [5].
The ability of the body cells to function depends on the ability of the heart to pump blood, the ability of the blood vessels to carry the blood for transportation of substances across capillary walls, the ability of the lymphatics to carry some of the interstitial substances back into the system, and the quantity and quality of the blood. The cardiovascular system requires a communication process to analyze how well it is performing its function in relation to the whole body, allowing it to determine priorities or make moment-to-moment adjustments. The vasomotor center (located in the pons and medulla oblongata) receives messages for the periphery and sends signals through the autonomic nervous system to the cardiovascular structures indicating what they are to do. These immediate adjustments are referred to as autonomic reflexes. To understand how these reflexes work, knowledge of the chemical mediators and the cardiovascular structural responses to them is necessary [2].
The postganglionic parasympathetic nerve fibers primarily secrete acetylcholine and are referred to as cholinergic. The postganglionic sympathetic nerve fibers, which secrete norepinephrine, are referred to as adrenergic. The sympathetic chemical transmitters to the sweat glands and a few blood vessels are cholinergic. This helps explain why sweating is increased with strong sympathetic discharge [2].
The heart is innervated by both parasympathetic and sympathetic nerve fibers. The parasympathetic fibers are primarily found in the atria, especially the SA and AV nodes; they are sparse in the ventricles. Parasympathetic stimulation of the heart results in a decreased heart rate (a negative chronoscopic response) and force of contraction (a negative inotropic response). Sympathetic fibers innervate the same areas as the parasympathetic fibers, but there is a greater number of sympathetic fibers in the ventricles. The heart responds to sympathetic stimulation by increasing its rate (a positive chronoscopic response) and increasing its force of contraction (a positive inotropic response). Sympathetic stimulation increases metabolism in the heart and creates the need for a greater blood supply [2,3].
The blood and blood vessels essentially respond to autonomic stimulation of the sympathetic nervous system. The blood responds to sympathetic stimulation by increasing coagulation. The blood vessels, including the arteries, arterioles, venules, and veins, respond by either constricting or dilating. However, the capillaries neither constrict nor dilate in response to sympathetic stimulations.
The heart and blood vessels have two types of adrenergic receptors: alpha and beta. Blood vessels with alpha receptors constrict when stimulated by adrenergic chemicals, while those with beta receptors dilate when stimulated by adrenergic chemicals. This helps explain why some vessels (such as those in the abdomen and skin) constrict during strong sympathetic stimulation, whereas those in other areas (such as the muscles) dilate [2,3].
The sympathetic nervous system affects the amount of blood circulating by influencing the heart rate and the force of cardiac contraction as well as by changing the diameter of the blood vessels. These factors influence blood pressure and peripheral vascular resistance, which in turn affects blood flow. Several peripheral receptors send messages to the vasomotor center, which promotes autonomic nervous system responses to regulate blood flow by influencing blood pressure. Some of these receptors are the baroreceptors, chemoreceptors, and atrial stretch receptors [2].
Baroreceptors are nerve receptors that respond to changes in blood pressure. This response triggers the adjustments needed when changing positions, such as from lying to standing. The baroreceptors are stimulated by increases in blood pressure and send signals to the vasomotor center, which inhibits vasoconstriction and stimulates a parasympathetic response. This results in less vasoconstriction, a slower heart rate, less force of cardiac contraction, and lower blood pressure. When blood pressure decreases, these receptors send fewer signals to the vasomotor center, causing more vasoconstriction, increased heart rate, and a greater force of contraction, which raises blood pressure [2].
The baroreceptors are found in most of the large arteries in the chest and neck. Of greatest clinical importance are the baroreceptors in the bifurcation of the carotid artery. When the heart rate is very fast, this area may be massaged, stimulating the baroreceptors to send more signals to the vasomotor center, indicating that they sense the blood pressure to be very high. The vasomotor center responds by decreasing the heart rate [2].
Chemoreceptors sensitive to arterial oxygen, carbon dioxide, and hydrogen ion concentration are located in the carotid bifurcation (carotid bodies) and at the aortic arch (aortic bodies). When the hydrogen ion and carbon dioxide content is high and oxygen content is low, these receptors send messages that excite the vasomotor center, resulting in sympathetic discharge in an effort to increase blood flow [2,3].
Stretch receptors in the atria send signals to the vasomotor center when the atrial pressure is high, indicating that blood is damming up in the heart. The vasomotor center responds by causing dilation of the peripheral arterioles, which results in less peripheral resistance and increases the ability of the heart to pump blood. When the arterioles to the kidneys dilate, not only is renal filtration increased, but the hypothalamus is signaled to decrease secretion of antidiuretic hormones and increase urine production. This effectively decreases the circulating blood volume and decreases the blood pressure [2,3].
The vasomotor center responds with a powerful sympathetic response when it does not have enough blood supply (i.e., when the systolic blood pressure falls below 50 mm Hg); the response becomes stronger as the blood pressure drops even lower. This is called the central nervous system ischemic response. The activated sympathetic nervous system stimulates the adrenal medulla to secrete norepinephrine and epinephrine, which causes the blood vessels and heart to activate the sympathetic nervous system responses. Other structures, such as the kidneys and hypothalamus, also influence vasomotor activity [3].
The blood pressure remains relatively stable over time (hours to days). The kidneys control longer term blood pressure and blood flow by regulating the body fluids [3].
Proper bone marrow functioning and overall integrity of the hematopoietic system are vital to survival. Many factors have been identified that contribute to the regulation of hematopoiesis. Tissue anoxia stimulates the production of erythropoietin, a hormone made in the kidneys and liver. Erythropoietin stimulates the bone marrow to increase red blood cell production. In addition, increases in altitude tend to increase erythrocyte production. At high altitudes, the amount of oxygen in the inspired air is diminished, which increases red blood cell production [3].
Erythropoietin production is also influenced by nutritional status, activity, age, chemical or radiation exposure, and drug therapy. Nutritional deficiencies in iron, vitamin B12, and folic acid tend to decrease erythrocyte production and interfere with red blood cell maturation. Overall, a sedentary individual has relatively less erythrocyte production. The sites of bone marrow activity change as one ages. In adults, the sites of bone marrow activity are confined to the pelvis, sternum, ribs, cranium, and ends of the long bones and vertebral spine. This decrease in functional bone marrow mass may account in part for the increased risk of anemia in the elderly population. Chemical and radiation overexposure may cause bone marrow damage and interfere with production of red blood cells, white blood cells, and platelets. Drug therapy of any kind can also influence the production of all blood cell components [3].
In addition to drug and radiation exposure, leukocyte production is also influenced by chemotactic substances during an inflammatory response. These substances signal the bone marrow to increase the release of white blood cells necessary for a normal inflammatory response. Lymphocytes continuously travel throughout the body, but their proliferation is stimulated during the invasion of viruses and foreign substances (antigens).
As with red and white blood cells, platelet production is influenced by overexposure to chemicals, radiation, and drugs. Hormones, especially estrogen and corticosteroids, tend to diminish the production of platelets [3].
As discussed, a major purpose of the circulatory system is to supply blood to the heart for recirculation. An adequate supply of blood is necessary for the body cells to function, and any problem that interferes with adequate blood supply may have serious consequences. Inadequate blood supply, or ischemia, usually produces pain. Temporary ischemia may do no damage; however, the cells may not be able to perform their functions as well as usual. Continued ischemia injures the cells. The injury may be reversible if blood flow increases sufficiently to meet the tissue demands, but if ischemia continues and irreversible damage occurs, the cells of the involved tissues or organs die. Tissue death is often referred to as infarction, necrosis, or gangrene.
Within physiologic limits, the heart pumps all the blood that returns to it. The difference between the cardiac output at rest and the cardiac output when the physiologic limit is reached (which may be more than four times the resting state) is called the cardiac reserve and varies according to the condition of the heart and factors that may interfere with the heart's ability to pump effectively. When the physiologic limits are exceeded, heart failure results. When the cardiac reserve is exhausted, the body tissues become ischemic. The heart itself also becomes ischemic, which may result in injury or death to some of its tissues and a further reduction in its ability to pump blood [10].
Cardiac reserve is essential for adjustment to the normal changes in body activity and stresses that increase tissue demands as well as to changes in the environment. During increased activity, the tissue demands increase as the cells require more oxygen and nutrients to function. When the environmental temperature is low, the cells increase their metabolic rate to maintain body temperature and therefore require a greater blood supply. When the humidity is high (reducing the effectiveness of sweating), blood is shunted to the skin to promote loss of body heat. Pregnancy also requires a much greater cardiac output than the nonpregnant state [10].
Cardiac reserve is reduced by factors that increase the workload of the heart or decrease the heart's ability to pump blood. The workload of the heart increases when cardiac output increases and when the heart has to pump against increased resistance. Factors that decrease the heart's ability to pump blood include those that impede or impair blood return to the heart and the heart's ability to work [1,10].
Some disease conditions increase the tissue demands for oxygen and nutrients, resulting in increased cardiac output. For example, hyperthyroidism is associated with increased cellular metabolism, which requires a greater cardiac output because the phagocytes have a high oxygen demand, and vasodilation occurs in the area of the affected tissues. In addition, these conditions raise body temperature, which promotes heightened metabolism and the need to release the body heat; this further increases the cardiac output [1].
Abnormal shunting of blood from arteries to veins, often the result of trauma or surgery, requires an increase in cardiac output because the shunted blood does not meet tissue demands for nutrients. These shunts, as well as abnormal congenital arteriovenous shunts, may not cause difficulties until combined with other conditions that put additional strain on the heart.
Another cause of increased cardiac output related to blood returning to the heart is volume overload. Increased blood volume may relate to increased fluid retention by the kidneys or overload of intravenous (IV) fluids [1].
The overall condition of the blood also affects cardiac output. Anemic states resulting from such conditions as blood loss result in the circulatory system being unable to carry enough oxygen and nutrients to meet cellular demands. Consequently, the cardiovascular system circulates the blood more often, increasing cardiac output. Increased output may also result from a lack of adequate oxygen or nutrients, as occurs in pulmonary disease, nutritional deficiencies, and liver disease [1].
Under some conditions, the heart pumps enough blood with each stroke, but some goes in the wrong direction. This happens with a ventricular septal defect after MI, characterized by blood being pumped from the left ventricle into both the aorta and the right ventricle. The same situation exists with incompetent valves that do not close tightly, causing tricuspid, mitral, pulmonic, or aortic regurgitation. In these cases, some of the blood flows back through the incompetent valve, and the heart has to pump the regurgitated blood again, increasing its workload [1,10].
When resistance to blood flow increases, the heart must create more pressure, increasing its workload and, subsequently, its need for more nutrients and a greater blood supply. With greater resistance, the total amount the heart is capable of pumping diminishes, affecting the amount of cardiac reserve. A common condition that places additional strain on the left ventricle is diastolic hypertension. (When the left side of the heart generates enough pressure to ensure flow into the aorta, it must overcome the pressure in the aorta for blood to flow.) Other factors that increase the resistance against which the left ventricle must pump include stenosis (narrowing) of the aortic valve or of the blood vessels from conditions such as coarctation of the aorta and atherosclerosis. Increased pulmonary resistance, which is observed with some lung diseases and pulmonic stenosis, increases resistance for the right ventricle. Other conditions that cause resistance to blood flow through the pulmonary vessels include pulmonary emboli that block some of the vessels and increased viscosity of the blood, which may occur with polycythemia vera, a disorder characterized by an increased number of erythrocytes [1,10].
Decreased blood return to the heart means the heart is unable to pump enough blood to meet the body's needs. Inadequate blood volume occurs with dehydration and massive hemorrhage. Insufficient blood return to the heart may be the result of massive vasodilation promoted by ineffective sympathetic control in conditions such as loss of nervous innervations by accident, surgery, or sympathomimetic drugs or chemicals. The constriction of the heart by the pericardium in pericarditis and pericardial tamponade also prevents sufficient blood from entering the heart. Mitral and tricuspid valvular stenosis inhibit ventricular filling during diastole and subsequently affect cardiac output. Disturbances in the heart's rhythm may also prevent the heart from having enough time to fill the ventricles completely [1,10].
Inadequate blood supply to the myocardium caused by diseases such as CHD impairs the heart's ability to pump blood and therefore reduces the cardiac reserves. Nutritional diseases of the heart as well as cardiomyopathies, infections, and trauma may also reduce the heart's ability to pump blood [1].
Blood flow through the vessels depends on the integrity of the structures and the ability of the vessels to perform their functions. To meet changing tissue demands for blood flow, the blood vessels change their diameters in response to local regulatory substances, temperature, vasoactive substances in the blood (e.g., vasopressin, angiotensin, epinephrine), and the sympathetic nervous system. The arteries stretch during systole to accommodate the blood pumped from the heart and recoil during diastolic to move it on. The veins depend on muscular movement and the action of their valves to move the blood. Disturbances in these functions result in the inability of the blood vessels to supply blood to the tissues properly, which can cause ischemia [1].
The blood vessels may lose integrity from trauma or disease processes that destroy their walls, resulting in bleeding. If the vessel is unable to contain the blood, it cannot fulfill its circulatory function. A common problem that inhibits the arteries' ability to stretch and recoil properly is atherosclerosis. In this disease process, atheromatous plaques, usually of lipid origin, develop in the tunica intima. The resultant inability to stretch and recoil places additional strain on the heart [10].
Over time, atheromatous plaques may become larger, progressively narrowing the arteries and causing atherosclerotic occlusive disease. As an artery narrows, it is unable to carry sufficient blood to the tissues it feeds, resulting in ischemia and tissue death. An affected artery may be able to handle enough blood during relative inactivity, when tissue demands are low, but when activity increases or tissue demands are higher, the narrowed artery is unable to provide enough blood flow and the tissues become ischemic. In addition, a hemorrhage may occur in the area of the plaque, causing formation of a blood clot and the complete blocking of the artery. This results in no blood flow and certain death of the tissues unless sufficient collateral circulation has developed [10].
Other diseases may obstruct blood flow in the arteries. Some obstruction results from inflammatory processes such as thromboangiitis obliterans, commonly called Buerger disease. Some diseases, such as Raynaud disease, cause arterial spasms that decrease blood flow and may result in ischemia. External pressures in excess of the arterial diastolic pressure inhibit arterial blood flow, and pressures that surpass the arterial systolic blood pressure stop blood flow though the artery. Common causes of obstruction to blood flow include tumors and swelling from injuries, such as burns. Clothing, bandages, or casts may tighten with swelling from bleeding or edema and obstruct blood flow. The constant weight of the body tissues on a vessel without intermittent relief of pressure may also prevent adequate circulation [10].
The inability of the veins to circulate the blood is called venous insufficiency. The most common cause is a faulty valve that promotes damming of blood in the veins. The hydrostatic pressure in the veins increases and causes edema in the tissue, from which the blood empties into the involved vein. Complications of chronic venous insufficiency include thrombophlebitis, hemorrhage, stasis dermatitis, stasis cellulitis, and stasis ulcers [3].
Any event that causes enough pressure to inhibit venous flow can contribute to venous insufficiency and result in edema. External pressure is commonly caused by garters or elastic used to hold up stockings or socks. Tumors or hematomas within the tissues may exert pressure against veins. Lack of the use of muscles that assist venous flow promotes venous stasis. Pressure on the veins from maintaining a constant position (e.g., crossing the legs at the knees) also inhibits venous flow [3].
An embolism is the obstruction of a blood vessel by a blood clot or foreign substance, such as air or fat. Emboli can occur in arteries or veins (or both). The most common type of embolus is a blood clot (thrombus) that forms in the heart or a blood vessel. The thrombus (or a piece of it) becomes dislodged and travels (then called a thromboembolism) until it arrives in a vessel so small that it cannot move any farther; it then blocks any flow ahead of it. If the embolus is in a vein (or the right side of the heart), it travels through larger and larger vessels until it arrives in the heart, where it moves through the right atrium and ventricle and into vessels in the lungs, which get smaller and smaller. When the embolus reaches a pulmonary vessel too small for it to pass through, it stops. It is then called a pulmonary embolus. When a blood clot breaks off in the left side of the heart or an artery, the thromboembolism travels through smaller and smaller arteries. When it comes to one smaller than itself, it stops and obstructs the blood flow ahead of it. The artery in which it stops may be anywhere in the systemic circulation (e.g., the brain, legs, arms, internal organs). The ultimate result may be death to the tissues ahead of it [3].
All systems of the body rely on the cardiovascular system and blood-forming organs to supply them with the blood needed to perform their functions. Thus, a problem with the cardiovascular or blood-forming system may result in devastating consequences for other parts of the body. The other body systems also influence the ability of the cardiovascular and blood-forming systems to perform their functions [11].
Without a healthy nervous system, the blood vessels are unable to respond to changes in a person's activity and position, and the baroreceptors, as well as the other reflexes, do not transmit messages to meet the moment-to-moment need for changes in blood flow. The temperature regulatory function of the cardiovascular system depends on communication between the vasomotor center and the hypothalamus, as well as the integrity of the skin [11].
The cardiovascular system is reliant on the respiratory system to make the necessary oxygen available and to remove carbon dioxide from the blood. Without the necessary oxygen, the cardiovascular system is not able to meet tissue needs. Lung-related problems may also increase the work of the heart, because they may promote increased resistance to blood flow as the heart responds to tissue hypoxia [11].
Without adequate intake of food and water and proper function of the gastrointestinal tract or the liver in metabolism (in processing and storing nutrients, as well as secreting substances necessary for digestion), the cardiovascular system is unable to deliver nutrients necessary for cellular function. The liver's formation of blood-clotting factors is necessary to sustain the integrity of the cardiovascular system [11,12].
Cellular health also depends on the ability of the liver and kidneys to excrete waste products. The failure of the kidneys to excrete excess water—whether due to a problem with the kidneys, hypothalamus, or pituitary gland—results in circulatory overload. This might stress the heart and promote an electrolyte imbalance, which would destroy blood cells [11,12].
The proper function of the musculoskeletal system is also essential to the ability of the bone marrow to produce blood cells. The most important role of the musculoskeletal system is the activity that returns blood to the heart for recirculation.
The endocrine system, through its secretions, affects the entire body metabolism, as noted with hyperthyroidism and its effect on increasing cardiac output. The role of the pituitary gland in secretion of antidiuretic hormone and the role of the adrenal glands in secreting aldosterone affect the cardiovascular system by regulating water and sodium balance. The adrenal glands are most important in the secretion of epinephrine and norepinephrine in times of need to assist the sympathetic nervous system in promoting vasoconstriction. The pancreas also plays a role in glucose metabolism [11,12].
A major physiologic problem caused by a disorder of the cardiovascular system, the blood, or the blood-forming organs is the inability to provide adequate nutrients to the cells. This disorder generally results in the patient's need to reduce the metabolic demands of the body by decreasing activity levels. The adjustment to decreased activity may be temporary, until the disease process is resolved, or it may involve a permanent change in lifestyle. A decrease in activity may not be compatible with the patient's life and responsibilities for home or career. For some persons, a planned exercise program as part of rehabilitation may also be difficult [13].
Patients may encounter other major physiologic problems with leukopenia and thrombocytopenia that require lifestyle changes. Individuals with leukopenia should take special precautions to prevent infection. Once established, infection can be fatal; therefore, patient education should be geared toward prevention. Patients may need to change their usual routine, especially during periodic leukopenic episodes. Avoidance of crowds or people with upper respiratory tract infections is also recommended. Even a simple trip to the grocery store may be contraindicated during leukopenic episodes. The patient and family or significant others may have difficulty coping with these changes [13].
Thrombocytopenia has its own set of restrictions to which patients must adjust. Patients with this condition require a knowledge of measures to prevent serious bleeding episodes during thrombocytopenic periods. Lack of knowledge about the prevention of infection or bleeding episodes may make the patient more prone to developing complications. Ideally, patients should take responsibility for learning about their disease processes and treatments. However, this may be too stressful for some individuals [13].
Together, changes in activity level, changes in nutritional habits, the need to take medications, and the added responsibility of learning about a disease process and its treatment require adjustments in developmental and cultural aspects of living. The nurse as counselor should assist patients by discussing their lifestyles in relation to the effects of the cardiovascular and blood-forming organs. Studies have shown that the level of compliance in both preventive and curative situations is low. A review of the literature found that about one-third to one-half of all patients studied did not follow suggested positive health practices [13]. Although not all patients will respond to recommendations, they have the right to information so they can make informed decisions about lifestyle changes [13].
Developmental tasks in adulthood include those related to their vocational, family, home, social, and leisure-time responsibilities. Depending on the capabilities of the cardiovascular system and blood-forming organs, patients' abilities to complete tasks may be impeded. For example, it is important to discuss the possibility of the patient's returning to work. The need for alterations in activities of daily living to accomplish the responsibilities in the home and participation in usual family activities also should be explored [13]. Activities such as sporting events and shopping trips may be too physiologically stressful or may put the patient at an increased risk for developing serious infections. In addition, some changes may be needed in living arrangements. For example, whether the patient will be required to climb steps is important [13]. Consider whether the patient will need to make changes in sexual activity.
Community activities should be assessed in relation to the need for energy expenditure and the possible risk of infection. Some activities may be stressful to the cardiovascular and blood-forming systems and should be eliminated. Others may be acceptable. Leisure-time activities also should be considered. Some people use their leisure time in sedentary activities and may need to make no adjustments during the recovery period. Others who participate in activities requiring a high energy expenditure may have difficulty adjusting. Some individuals who have no hobbies or leisure-time activities find having time on their hands stressful [13].
In some cases, eliminating an activity may be more stressful to patients than establishing a new leisure regimen. Probably the most important issue in counseling the patient about a change in activity level is the importance the activity has for the patient. Some activities may need to be eliminated, some may be continued if they are spaced, and some may need no adjustment at all.
A person's beliefs and values play a significant role in the development of cardiovascular diseases as well as in his or her compliance with rehabilitation and treatment for both cardiovascular and blood disorders. The role of personality in heart disease has received considerable attention. The type D personality, defined as a distressed personality characterized by high levels of both negative affectivity and social inhibition, may be more prone to MI than other personality types [14]. Although emotional stress is neither good nor bad, observations indicate that it does have a physiologic effect on heart rate, blood pressure, and respiration. The extent to which a person is stressed is an individual matter, and the effect of the stressor is related to its significance to the individual [13].
The culture in which a person lives greatly determines the significance of emotional and physiologic stressors. For example, smoking has been a culturally acceptable behavior identified as a major risk factor in the development of cardiovascular disease. The importance a person places on such areas as family, work, eating, social activities, and health care is also related to cultural values.
Diet is an important factor in all diseases because without the proper nutrients, the cells cannot function properly. Certain dietary habits are associated with cardiovascular disease. For example, high blood cholesterol is a major risk factor in the development of atherosclerosis, and patients can control blood cholesterol levels by adhering to a diet low in saturated fat and cholesterol. Weight loss can similarly improve cardiovascular health. Changing the dietary habits of one member of the family usually effects changes in the diets of others as well, which may place stress on the entire family but also has the potential to improve the entire family's health [15].
Changes in diet, occupation, activity level, and living arrangements, as well as the need for medication and the cost of health care, may cause an economic burden for the patient and his or her family. In addition, persons with cardiovascular or blood disorders may have difficulty obtaining insurance. Consequently, insurance costs may be prohibitive.
The patient with a cardiovascular or blood disease may or may not be able to return to work. Alternatively, job requirements may require adjustments, which can produce stress between the patient and peers over workload. Workers' compensation insurance may be available if the cardiovascular problem is related to the job, and in cases of complete disability, the patient may be eligible for Social Security compensation.
Environmental threats to the person with cardiovascular and blood problems include terrain, altitude, and climate. For example, climbing an incline requires more energy expenditure than walking on level ground. Environmental temperature extremes place an additional burden on the cardiac and vascular systems. Cold temperature promotes vasoconstriction and shivering to generate body heat, and a person with an already diminished blood flow may experience ischemia. Shivering increases the body's metabolic needs and cardiac output. Hot temperatures promote dilation of the vessels near the skin, which also requires an increased cardiac output [13]. A significant increase in altitude results in increased cardiac output, tachycardia, and possibly a slight increase in blood pressure, but no change in stroke volume [16].
Obtaining a cardiovascular health history is the first and most important step in cardiovascular assessment. Selection of diagnostic tests, interpretation of test results, and prescription of therapy all depend on the patient's history. Many patients fear heart disease and understate their symptoms. By carefully observing nonverbal gestures, reactions to questions, the words used to describe symptoms, and overall attitudes, the nurse can evaluate patients' emotional responses. Assessment of a patient's emotional support system is also beneficial, especially if invasive procedures must be performed or long-term disability is a possible outcome. Taking the time to get to know patients and their families or significant others helps the nurse to correlate behavior and emotional factors with the onset of cardiac symptoms.
The principal symptoms of heart disease are dyspnea, chest pain, palpitation, and syncope. Other common symptoms are fatigue and cough. The nurse should attempt to gain information about these symptoms, evaluating them according to the level of activity that precipitates the symptom. Patients may not experience any problems with minimal activity, but the symptoms may appear with exertion. This is the classic situation with cardiovascular dysfunction [17].
Dyspnea
Dyspnea, the most common symptom of both cardiac and pulmonary diseases, is defined as difficult or labored breathing. Patients may report that it is difficult to breathe, that there is not enough air, or that they become short of breath with any activity. In heart disease, dyspnea usually occurs with exertion and patients report being unable to get enough air into their lungs. In pulmonary disease, dyspnea occurs both at rest and with exertion and patients feel more difficulty during exhalation. The differentiation between cardiac and pulmonary dyspnea is difficult; the two diseases often coexist.
Dyspnea should be evaluated according to the extent of activity it takes to induce the symptom. Ask patients if they can perform their normal daily activities without difficulty in breathing. If they have no problems with these activities, ascertain the highest level of activity they can attain without dyspnea (e.g., the ability to climb two but not three flights of stairs without difficulty). Dyspnea associated with exertion is known as dyspnea on exertion and is an early symptom of CHF [18].
Paroxysmal Nocturnal Dyspnea
Paroxysmal nocturnal dyspnea occurs during sleep. The patient usually awakens suddenly, breathing with difficulty and having a sensation of suffocation. This usually occurs two to five hours after the onset of sleep and happens only once during the night. Paroxysmal nocturnal dyspnea occurs in patients with CHF and is due to pump failure of the left ventricle, which leads to fluid accumulation in the lungs. It is relieved by sitting upright with legs over the side of the bed or by walking around the room. It usually subsides within 20 minutes without aftereffects, and the patient can sleep the remainder of the night [18].
Orthopnea
Orthopnea is a form of dyspnea that develops when the patient lies down and is an indicator of left ventricular failure. It is relieved within minutes by sitting up or standing. Patients with orthopnea often require several pillows at night to elevate the head and prevent nocturnal breathlessness. In fact, the severity of the condition was often informally measured by the number of pillows necessary. As heart disease advances and CHF progresses, the severity of this symptom increases. In severe heart failure, the patient is unable to lie down and usually sleeps in a chair. To obtain information about this symptom, ask the patient how well she or he sleeps, and determine how many pillows the patient normally uses and whether the number necessary to provide breathing comfort during the night has increased [18].
Chest Pain
By far the most frightening cardiovascular symptom for patients is chest pain. Because chest pain may have a variety of causes, the main objective is to determine the origin. The most common origins are cardiac, pleuropulmonary, musculoskeletal, gastric, or psychosomatic. Pain originating from the heart is due to ischemia, and ischemic pain is referred to as either angina or MI pain. Diagnosis depends on the patient's history and subsequent physical examination. The most serious cause of chest pain is MI. As such, this should be considered first, and when the diagnosis of MI is ruled out, then questioning can be directed to other causes. When obtaining information about chest pain, assess the following aspects: the onset, location, radiation, duration, quality, alleviating and aggravating factors, and associated symptoms.
Chest pain due to MI is often described as a crushing substernal pain. The patient may say, "It felt as if a ton of bricks was on my chest." A common gesture patients use is a tight fist over the center of the chest. The pain is usually associated with dyspnea, diaphoresis, and (less frequently) nausea and vomiting. The onset is acute and not associated with a precipitating event. The pain lasts longer than 5 minutes; nothing relieves it. Often, the pain radiates to the left or right arm, into the neck, or to the jaw or back. Occasionally, the patient may have only radiating pain [13,18].
Like MI pain, angina is a constricting, pressure type of pain. Angina differs from the pain of an MI in that it is episodic and temporary, usually lasting less than 5 minutes. Also, the onset is associated with exertion, emotion, eating, or exposure to cold. Acute episodes are relieved by nitroglycerin and/or rest. For long-term management, angina is controlled by medications, usually a beta-adrenergic inhibitor (beta blocker). If angina is uncontrollable and the patient is unable to maintain an adequate lifestyle, coronary bypass surgery may be required.
Other serious causes of chest pain are pulmonary embolus and dissecting aortic aneurysm. Pain from a pulmonary embolus is described as stabbing, shooting pain. The pain increases with inspiration and is often associated with a sudden onset of dyspnea, tachycardia, hypotension, diaphoresis, rales, and hemoptysis [13,18]. Pain from a dissecting aortic aneurysm is characterized as a sudden, tearing, intense chest pain with radiation to the back, flanks, and legs. Usually, the patient has a history of hypertension.
Chest pain well localized to a specific area of the chest wall is usually due to a musculoskeletal problem. Tenderness in response to palpation is often present. An example is chest pain due to inflammation of the costochondral junctions of the ribs and sternum, or pleurodynia, a rare complication of coxsackievirus B infection [13,18].
Stress and anxiety may also cause chest pain. This type of pain is usually localized in the left chest wall and does not radiate. It may be described as suffocating pressure. The history assists in determining whether the chest pain is related to stress and anxiety.
Palpitation
Palpitation is an unpleasant awareness of the heartbeat. The patient often describes a fluttering feeling in the chest or says that the heart seems to jump, race, pound, stop, or skip beats. Palpitations are most often due to rhythm disturbances such as premature contractions, atrial fibrillations, or sinus tachycardia. However, anxiety, stress, fatigue, or cardiac stimulants (e.g., caffeine, nicotine) are also factors that can precipitate palpitations. Detailed questions about the onset, relation to exercise, presence of associated symptoms (e.g., dyspnea, syncope), and relieving factors (e.g., stooping, breath holding) assist in determining the significance of the palpitation. Drug toxicity may also cause palpitations. Therefore, a medication history, which includes asking how the patient takes the medication, is necessary. Be certain to include a history of over-the-counter medications as well as prescribed drugs. Cold remedies, such as cough suppressants, commonly cause palpitations [13,18].
Syncope
Syncope, a transient loss of consciousness, is associated with muscle weakness and an inability to stand. It is due to inadequate blood flow to the brain, which may be a result of a cardiac rhythm disturbance or decreased cardiac output from valvular disease. Dizziness with an inability to maintain an upright posture is referred to as near syncope. The patient should describe the circumstances that lead to dizziness or fainting. Syncope that occurs with exercise may be related to aortic or subaortic valve stenosis. This condition is serious and requires further documentation, usually through noninvasive testing.
A sudden loss of consciousness due to a heart block is known as a Stokes-Adams attack. This type of syncope commonly occurs in elderly patients and is followed by breathlessness and an absence of pulse, usually lasting only seconds. Cardiac arrest may occur if respiration and circulation are not restored.
The elderly patient may also develop another type of syncope caused by hypersensitivity of the carotid sinus bodies. The carotid sinus bodies are located in the carotid artery below the jawline. Pressure applied to the carotid artery may stimulate a vagal response that decreases the blood pressure and heart rate; exaggerated vagal response may produce syncope. The patient may report episodes of fainting while shaving or buttoning a tight collar. Digoxin appears to increase carotid sinus sensitivity, making the patient more susceptible to syncope. Bilateral palpation of the carotids should never be performed [13,18].
Fatigue
Fatigue is a frequent complaint of patients suffering from cardiovascular dysfunction. Patients describe muscle weakness and an inability to complete normal daily activities, often requiring naps to function. The fatigue is probably related to a combination of physical and emotional factors. Physically, the fatigue is thought to be related to insufficient blood flow to the tissues, the result of inadequate cardiac output, while depression is the primary emotional factor. Fatigue is assessed by the level of activity tolerance [13,18].
Cough
Cough associated with cardiovascular disease is due to fluid accumulation in the lungs and is described as dry, irritating, spasmodic, and nocturnal. Patients may cough after episodes of dyspnea. A productive cough with colored sputum may indicate a pulmonary infection [18].
Psychosocial Response
A serious disruption of cardiovascular function can be a significant emotional and physical threat. The onset of heart disease is often considered a major life crisis. The life of a patient who has a cardiac event (an MI or cardiac surgery) may be either immediately threatened or altered. Survival cannot be guaranteed, and future plans often need revision. Former support systems may be diminished, and roles as spouse, parent, and worker may be interrupted. Central to this crisis is the fear of death, pain, disability, and physical dependency.
Many patients respond to these fears by developing anxiety [13,19]. Anxiety may also be a result of the patient's assessment of personal body image. An acute event poses a great threat to body integrity and function. The personal meaning of the heart to the patient and the impact of the event on body image will greatly influence the patient's convalescence. Patient role responsibilities, knowledge of the severity of the disease, and the reaction of significant others are important components to assess.
To cope with anxiety and fear, the patient may use denial. In the acute care setting, denial is a common defensive coping mechanism and serves to protect the patient from perceived threats. As an effective coping mechanism that decreases the physical and emotional outcome of anxiety, denial is beneficial in the acute phase of illness. Sustained denial, however, is maladaptive. The nurse should assist patients in identifying effective coping mechanisms to prevent them from developing a crippling psychological state [13,19].
After obtaining information about the patient's symptoms, review his or her past health history, paying particular attention to information about previous hospitalization(s) for cardiac problems. If cardiac surgery was performed, document the date, the type of surgery, number of bypasses, and any complications. If valvular surgery was performed, include the type of valve used. If a cardiac pacemaker was implanted, the patient should know the type and have the model number available. Previous diagnostic procedures performed, such as ECGs, exercise stress testing, or cardiac catheterizations, add to the patient's history. Certain childhood diseases predispose the patient to cardiovascular dysfunction and should be noted. Childhood rheumatic fever can cause valvular disease that becomes evident in adulthood. Untreated streptococcal infections may also cause valvular disease. Maternal exposure to rubella in the first two months of pregnancy is associated with congenital heart defects in offspring. Many children who have had corrective surgery for congenital heart defects are now living through adulthood; therefore, information regarding childhood cardiac problems should be obtained [17].
A medication history, including prescribed and over-the-counter medications, should be obtained. As noted, cardiac symptoms may be precipitated by some over-the-counter drugs, including many antihistamines and decongestants, and antitussives contain sympathomimetic amines, which may cause palpitations for transient hypertension. Some antacid preparations contain large amounts of sodium, which may cause fluid retention and a subsequent increase in blood pressure. If the patient is taking a prescribed medication, ask how he or she is taking it, specifying the time of day and the amount.
A dietary history can assist in evaluating the patient's understanding of the relation between food and heart disease. Assess the patient's intake of red meat, salt, dairy products, and sugar, and estimate total daily calories. Evaluate the patient's weight compared with the recommended ideal weight [17].
An important component in cardiovascular assessment is evaluating the patient's cardiovascular risk factor profile. Risk factors are personal characteristics and habits that increase the patient's chances of developing CHD and may be categorized as unalterable or alterable. Unalterable risk factors include sex and a family history of heart disease or hypertension. Alterable risk factors include hypercholesterolemia, smoking, physical inactivity, obesity, alcohol consumption, and oral contraceptive use. Personality may also contribute to CHD. As discussed, type D behavior has been associated with twice the normal risk of developing CHD [14]. The most prominent risk factors are hypertension, a high blood cholesterol level, and cigarette smoking [17].
Hypertension
Unequivocally, hypertension, either systolic or diastolic, increases the risk of developing CHD; the higher the blood pressure, the greater the risk. Hypertension causes coronary, cerebral, and renal vascular disease and is the leading global cause of death and disability among adults [20].
Symptoms attributed to hypertension may include headache, epistaxis, tinnitus, dizziness, and fainting. Unfortunately, complicated hypertension is usually asymptomatic until significant organ damage occurs. The U.S Preventive Services Task Force recommends adults 40 years of age and older and younger persons at increased risk for high blood pressure be screened annually [21]. Adults 18 to 39 years of age with normal blood pressure (less than 130/85 mm Hg) who do not have other risk factors should be rescreened every 3 to 5 years [21].
Cholesterol
In evaluating cholesterol, ask about the patient's previous history of elevated cholesterol levels and any therapeutic and preventive regimens the patient is following. Ascertain how much the patient knows about the role of cholesterol in developing CHD and whether she or he understands the role of diet, exercise, and weight in controlling cholesterol levels. A diet low in saturated fats and high in polyunsaturated fats is beneficial. A reduction in calories is usually recommended, especially if the patient is overweight. Aerobic exercise appears to increase high-density lipoprotein (HDL) levels, which appear to impede the development of CHD.
The American College of Cardiology/American Heart Association guideline for the treatment of blood cholesterol to prevent cardiovascular disease does not set cholesterol targets and instead focuses on the patient's overall risk for developing heart disease in the next 10 years (if not already present). That said, preventive therapy is recommended for all persons with low-density lipoprotein (LDL) cholesterol levels of 70 mg/dL or greater [22]. Patients should be advised to limit their saturated fat intake (e.g., tropical oils, butter, shortening, lard) to less than 6% of daily calories, as saturated fat intake is one of the most significant contributors to the production of cholesterol in the body [23].
Smoking
The principal cause of death in cigarette smokers is CHD, not lung cancer, and the risk is directly related to the number of cigarettes smoked per day. Smoking one pack or more each day increases the risk of heart disease at least threefold. The duration of smoking history and whether smoke is inhaled is significant. In addition, when cigarette smoking is combined with oral contraceptives, the risk of CHD and MI is extremely high. Smokers who have hypertension and/or diabetes mellitus are also at greater risk [19].
The risk can be reduced by discontinuing smoking. Within one year, the former smoker halves their risk of CHD [24]. The patient should understand this fact in order to encourage smoking cessation.
After explaining the effects of cigarette smoking, offer the patient assistance in smoking cessation. Group classes are effective, but if the patient prefers individual help, the American Lung Association provides a written program [25]. The health provider should carefully review this program with the patient. Informing the patient of other community resources increases the patient's chances of smoking cessation. In addition, there are pharmacotherapeutic options to assist patients taking steps to quit smoking. The first-line pharmacologic interventions for smoking cessation are nicotine replacement therapy, bupropion, and varenicline.
Physical Inactivity
Exercise appears to be effective in preventing CHD by reducing other risk factors such as stress, obesity, and elevated cholesterol levels. Numerous studies have documented the benefits of exercise as an effective strategy for both primary and secondary prevention of heart disease. It has been demonstrated that regular exercise reduces the risk of both overall mortality and cardiovascular mortality [26]. Moreover, patients with established heart disease show improved activity tolerance and quality of life after beginning an exercise program. Although approximately 4% to 15% of heart attacks occur during or soon after exertion, it is the least active patients who are at greatest risk. The benefits of exercise are far greater than the cardiac risks, and sudden cardiac death from exercise is extremely rare [26].
The recommendation by the Institute of Medicine is for adults to set a long-term goal of at least 60 minutes per day of moderate-intensity physical activity (e.g., brisk walking) or shorter periods of more intense daily activity [27]. Patients should be encouraged to incorporate exercise into their lifestyle as a routine. Walking is usually recommended as an easy type of exercise that can be done anywhere. The amount and duration of exercise are prescribed after patients undergo a complete history and physical examination [28].
Alcohol Use
Many researchers have replicated the finding that moderate alcohol consumption is associated with a reduced risk of CHD, peripheral artery disease, sudden death, and stroke and suggest that this effect is to a large extent mediated by increases in HDL [29]. A 2011 meta-analysis inclusive of 84 out of 4,235 studies on the benefits of alcohol concluded that the lowest risk of CHD mortality was conferred by one to two drinks per day and that the lowest stroke mortality risk was conferred by consuming one or fewer drinks per day [30].
A 2018 meta-analysis assessed alcohol consumption across three periods in each cohort to determine participants' intake trajectories over approximately 10 years [31]. The results indicate that instability in drinking behaviors over time is associated with increased risk of CHD. Compared with consistently moderate drinkers (i.e., men who consumed 1–168 g ethanol/week or women who consumed 1–112 g ethanol/week), inconsistently moderate drinkers had a significantly greater risk of incident CHD. An elevated risk of incident CHD also was found for former drinkers and consistent nondrinkers. Only former drinkers had a significantly elevated risk of fatal CHD outcomes. There was no elevated CHD risk for consistently heavy drinkers and only a weak association with fatal CHD for inconsistently heavy drinkers [31].
Research suggests that the protective effect may be a result of an interaction between diet and genetics, specifically related to a genetic variation in alcohol dehydrogenase (ADH) [32]. Moderate drinkers who are homozygous for the slow-oxidizing ADH3 allele have higher HDL levels and a substantially decreased risk of MI [32]. An acute protective effect of alcohol consumption was also found for regular drinkers who consumed one or two drinks in the 24 hours preceding the onset of cardiac symptoms. Risk of a major coronary event is lowest among men and women who report daily drinking. Alcohol does have effects on several markers for coronary risk factors, such as blood pressure, HDL cholesterol, LDL cholesterol, fibrinogen, clotting factors, and insulin sensitivity. However, excessive alcohol consumption increases cardiovascular risk factors and mortality. Documentation of alcohol intake should include the patient's pattern of drinking as well as the amount and type of alcohol consumed.
Caffeine Consumption
Caffeine is a cardiovascular stimulant that can cause tachycardia and dysrhythmias and may contribute to other risk factors such as hypertension. Determine the patient's total intake of caffeine, including coffee, tea, chocolate, energy drinks, and soft drinks. Consuming more than 400 mg per day is often considered excessive. Some patients who are especially sensitive to caffeine may experience symptoms with lesser amounts [33].
Physical assessment of the cardiovascular system begins with a general examination of the patient. Cardiac dysfunction may directly affect other body systems, and likewise, systemic illnesses often have cardiac manifestations. For these reasons, a thorough examination is necessary.
First, observe the general appearance of the patient, including physical build, skin color (e.g., pallor or cyanosis), and the patient's emotional status. Tall patients with long extremities and arm spans exceeding their height may have Marfan syndrome, which is associated with a variety of cardiac disorders.
Assess whether the patient appears to be in pain. Typically, the patient with angina sits quietly, whereas the patient with acute MI pain is uncomfortable and moves continuously. With pericarditis, the patient often assumes a sitting position, leaning forward.
It is also important to note chest contour. Some thoracic deformities, such as kyphoscoliosis, pectus carinatum, and pectus excavate, can affect the position of the heart and possibly cardiac functions [17].
The general description is followed by a detailed inspection. Abnormal facial appearance, particularly facial edema, color, and skin texture, should be noted. Rheumatic heart disease with severe mitral stenosis may cause cyanotic lips and jaundice. Constrictive pericarditis causes fluid retention, which can result in swelling of the face.
A fundoscopic exam is performed on all patients. Changes caused by hypertension, such as AV narrowing, exudates, and hemorrhage formations, are seen on fundus. A thin, grayish-white circle, an arcus, in the iris may be apparent in patients with hypercholesterolemia.
Skin color and temperature are important indicators of circulation. Pallor is the absence of the normal pink/rosy undertone, and one cause of pallor is vasoconstriction, which decreases blood flow to the skin. In patients with deeply pigmented or dark skin, the conjunctivae and oral mucosa are examined for skin color changes.
Cyanosis, a blue tinge (or a grey tinge in persons with darker skin) to the skin that appears when hemoglobin oxygen saturation is reduced, can be central or peripheral. Central cyanosis is assessed in the lips, mucous membranes, and nail beds; peripheral cyanosis is found in the extremities. With prolonged central cyanosis, clubbing of fingers and toes can occur.
The nails are a good source of information regarding cardiovascular status. Circulation can be evaluated in the nail beds by applying pressure to the distal part of the fingernail and noting the pallor as the capillary blood flow is temporarily halted. When the fingernail is released, the color should be restored within one to two seconds, or by the time the words "capillary refill" may be said. The nail beds should also be inspected for hemorrhagic areas resembling splinters, common in patients with bacterial endocarditis.
The temperature of the skin and extremities also reflects circulation. Cool, pale, wet skin often indicates a decreased skin circulation. This condition is seen in patients with acute MI when the cardiac output is suddenly reduced [17].
Edema is a local or general accumulation of excess fluid in the body tissues, and its presence in any dependent area (e.g., the extremities, sacrum, abdomen) is important to recognize. Edema is categorized as either pitting or non-pitting. Pitting edema is considered the more serious of the two, but the presence of either type is significant. The depth of pitting and the extent and location of edema are indicators of the severity of the condition. Some cardiovascular causes of bilateral edema include CHF and constrictive pericarditis. Inquire if the patient's shoes, rings, or clothes are becoming uncomfortably tight, as this may reflect fluid retention. Changes in body weight also reflect fluid status. A weight gain in a short period may be due to fluid retention, and sudden increases in weight may indicate cardiac failure [17].
The rate and quality (rhythm and force) of arterial pulses provide significant information regarding cardiac output. Pulses should be examined bilaterally and include the carotid, brachial, radial, ulnar, femoral, popliteal, dorsalis pedis, and posterior tibial pulses. A pulse rate greater than 100 beats per minute (bpm) (also known as tachycardia) is considered abnormal for healthy adults. Although a variety of factors may cause tachycardia, the long-term effect is usually decreased cardiac output. A pulse rate of less than 60 bpm (bradycardia) is also considered abnormal, except in patients with well-conditioned hearts (e.g., marathon runners). In the diseased heart, bradycardia causes a decrease in cardiac output, possibly indicating heart block.
The quality or force of the pulse is assessed on a scale of 0 to 4, with 0 equal to impalpable or absent and 4+ equal to full and bounding; a normal pulse is designated as 4+ [17]. An irregular pulse is associated with cardiac dysrhythmias.
The absence of a pulse may be a normal variation, particularly in the popliteal, ulnar, or posterior tibial pulses. A diminished or absent carotid pulse, however, usually indicates arterial disease. When a pulse is not palpable, a more distal pulse is assessed. For example, the dorsalis pedis pulse is assessed when the popliteal pulse is not palpable, and the radial pulse is assessed when the brachial pulse is not palpable. If distal pulses are felt, adequate circulation is present. If absent, other assessment parameters should be noted, such as skin temperature, skin color, and sensation.
Pulses are assessed bilaterally (i.e., right radial and left radial). Asymmetric or unequal bilateral pulses are abnormal and may indicate a serious circulation problem. One exception is the carotid arteries, which should never be palpated bilaterally. Palpation can overstimulate the pressure sensors (carotid sinus bodies), which will decrease heart rate and may result in syncope, particularly in elderly patients.
The arteries are also assessed by auscultation. Arteries that have some occlusion, causing turbulence of blood flow, will result in bruits. A carotid artery bruit may be due to occlusion or may be a referred systolic murmur from aortic valve disease [17].
The quality of the pulse may also be assessed according to the shape of the waveform, which can be palpated or displayed and measured by an arterial pressure monitor. Pulse waves that alternate in strength, with every other beat being weaker than the preceding beat, are known as pulsus alternans. This condition is common in patients with severe arterial hypertension and/or left ventricular failure. A weak pulse that seems to have a delay in the beginning (upstroke) and a delay in the ending (downstroke) is pulsus parvus and is indicative of aortic stenosis, mitral stenosis, constrictive pericarditis, or cardiac tamponade. A strong, bounding pulse with a rapid upstroke and downstroke, known as pulsus magnus, is seen in patients with hypertension, thyrotoxicosis, or aortic insufficiency. A double-beating pulse, called pulsus bisferiens, is an indicator of aortic insufficiency and aortic stenosis [9,34].
The effect of respiration on the pulse pressure can be assessed by use of the sphygmomanometer. Normally, the pulse pressure decreases approximately 10 mm Hg with inspiration; a drop of more than 10 mm Hg is considered abnormal. This condition, referred to as a pulsus paradoxus, is often found in patients with pericardial effusion, constrictive pericarditis, or severe pulmonary emphysema [34].
Blood pressure is routinely assessed in all patients. The indirect method is typically used, employing a sphygmomanometer and stethoscope. However, there is growing evidence supporting the use of automated measurement devices [35]. These newer oscillometric devices automatically inflate multiple times (in one- to two-minute intervals), allowing patients to be alone and undisturbed during blood pressure measurement. Guidelines published by the American College of Cardiology and the American Heart Association provide a checklist for accurate measurement of blood pressure [35].
Blood pressure is a good indicator of cardiovascular health and reflects both the patient's physical state and psychological effects on his or her physical state. Blood pressure is initially obtained in both arms with the patient in supine, seated, and standing positions [35]. The blood pressure in both arms should be comparable; a difference of more than 10 mm Hg is considered abnormal. Venous pressure is estimated by inspecting the jugular vein and measuring the height of pulsation from the sternal angle.
Korotkoff sounds (arterial vibrations) are used to measure blood pressure. As the blood pressure cuff is deflated, note the onset of the first sound, the muffling point of the sound, and the disappearance of the sound. The onset of the first sound is the systolic pressure; the muffling point and the disappearance of sound have both been used as the diastolic pressure reading. In healthy adults, the cessation of sound may best approximate the diastolic pressure. In hypertensive patients, there may be a silent interval between the systolic and diastolic pressure, known as the auscultatory gap. Noting the muffled sound may be more appropriate in these patients.
Cuff size is an important factor in obtaining accurate blood pressure. The cuff should be 20% wider than the diameter of the arm and cover two-thirds of the upper arm. If the cuff is too small, the reading will be elevated. Conversely, a cuff that is too large will artificially lower the reading. Positioning of the cuff is also important. The bladder of the cuff should be centered over the brachial artery, with the lower edge 1 to 2 inches above the antecubital space. The arm should be positioned so that the brachial artery is at the level of the heart. If it is below that level, the blood pressure measurement will be artificially increased; if it is above that level, the measurement will be artificially decreased.
Patients with a history of vascular disease or hypertension should also have initial blood pressure readings performed on both legs. In patients taking medication that affects blood pressure, the timing of blood pressure measurements in relation to ingestion of the medication should be standardized, and an average of two to three measurements taken on separate occasions should be obtained, thus taking into account random or unpredictable variations in measurements [35]. Blood pressure in the legs is normally higher than in the arms. Lower blood pressure in the legs may indicate an abdominal aortic obstruction or coarctation of the aorta. A large cuff is placed around the thigh, and the popliteal artery is auscultated. Leg blood pressure is best obtained with the patient in the prone position.
Body position may have a significant effect on blood pressure in patients who take diuretics or who have a fluid volume deficit. A slight drop in systolic pressure (less than 10 mm Hg) upon standing is normal, but a drop in diastolic pressure is abnormal.
Hypertension is diagnosed in the presence of systolic blood pressure of 140 mm Hg or greater and/or diastolic blood pressure of 90 mm Hg or greater [35,36]. Systolic hypertension is often seen in elderly patients, usually due to atherosclerosis. Increased cardiac output from conditions such as thyrotoxicosis, anemia, anxiety, and aortic regurgitation can also cause systolic hypertension. Diastolic hypertension is associated with essential hypertension, arteriosclerosis, and renal disease. Low blood pressure or hypotension may be seen in patients with MI, shock, or hypovolemia.
Pulse pressure, defined as the difference between the systolic and diastolic pressures, is also an indicator of cardiovascular states. The normal pulse pressure is between 30 mm Hg and 40 mm Hg. A value greater than this is referred to as a widened pulse pressure, which may occur in conditions such as hypertension, aortic regurgitation, deteriorating neurologic conditions, and thyrotoxicosis. A pulse pressure less than 30 mm Hg is called a narrowed pulse pressure and may occur in shock, pericardial effusion, severe aortic stenosis, or constrictive pericarditis.
In addition to the basic cardiac assessment and evaluation of the neck veins, certain other factors should be assessed. During inspection, observe the precordial pulsations in the sternoclavicular, aortic, pulmonic, right ventricular, apical, epigastric, and ectopic (overlaying the wall of the left ventricle) areas. Ventricular dysrhythmias, such as premature ventricular contractions (PVCs), may be seen as pulsations in the ectopic area [37,38].
After inspection, palpate the same precordial areas to confirm the previous observations. If there is a pulsation, evaluate its location, size (e.g., diffuse or discrete), timing, and duration in relation to the cardiac cycle. Note the presence of low-frequency, purring vibrations, referred to as thrills. Thrills are associated with valvular disease and can be felt especially with aortic stenosis. The timing of the thrill may be systolic, diastolic, or both [37].
During auscultation, a normal physiologic splitting of the first heart sound (S1) and the second heart sound (S2) may be heard. The simultaneous closure of the mitral and tricuspid valve is heard as S1. The normal splitting of S1 is more difficult to hear, although it may be heard occasionally in the tricuspid area. An easily heard S1 splitting is abnormal. Often, the sound is a diastolic S4 heart sound [37,38].
The S2 is a physiologic split sound that results from differences in the intracardiac pressures in the left and right heart. The split can be augmented by having the patient breathe deeply. A normal physiologic split occurs only during inspiration. S2 splitting that disappears with inspiration is referred to as paradoxical splitting and may signify heart disease. Fixed splitting (i.e., wide splitting during both respiratory cycles) is often seen with atrial septal defects and right ventricular failure. A high-pitched sound occurring after S2, very early in diastole, is known as an opening snap. It is heard best at the apex with the patient in the left lateral decubitus position. An opening snap is often associated with a stenotic mitral or tricuspid valve. Occasionally, an opening snap may be heard in patients with a high cardiac output [37,38].
Extra heart sounds heard during systole (between S1 and S2) are referred to as clicks. Clicks are high pitched and may be heard at any auscultatory site during early, middle, or late systole. Generally, there are two types of clicks: ejection and nonejection. An ejection click occurs in early systole as the blood rushes through the semilunar valves and into the aortic and pulmonic arteries. The sound is thought to be caused either by bulging of a stenotic valve or vibrations in a dilated vessel wall. The nonejection click occurs in middle-to-late systole. It is high pitched and can be heard best in the mitral area. The sound is associated with prolapse of the mitral valve. All clicks are abnormal, but the severity varies with each patient [37,38].
Patients with pericarditis may develop a friction rub. The sound usually occurs throughout the cardiac cycle, but it appears to have three components corresponding to atrial contraction, ventricular contraction, and ventricular filling. With inflammation, the friction rub is produced by the two layers of the pericardial sac rubbing against each other. The sound is scratchy, like sheets of sandpaper rubbing together or like new leather creaking. The rub is high pitched and heard best with the patient leaning forward. The location is variable but often heard best at Erb's point or the third left intercostal space. Rubs may be signs of pericarditis or pericardial infections [37,38].
Murmurs heard in midsystole or throughout systole are usually associated with valvular disease. Midsystolic ejection murmurs are caused by either pulmonary or aortic (often the result of rheumatic fever or congenital disease) stenosis. Murmurs associated with aortic stenosis are best heard in the aortic area (second right intercostal space), with the patient sitting upright. The murmur is high pitched and blowing and radiates to the neck and apex. Older adults often have calcification of the aortic valve, which can cause a murmur [37,38].
Pulmonary stenosis is less common than aortic stenosis but, when present, may be associated with other congenital defects, such as tetralogy of Fallot. In adults, the pulmonic systolic ejection murmur is usually due to pulmonary hypertension. This murmur is high pitched and harsh, is best heard at the second and third left intercostal space and radiates toward the left shoulder.
Murmurs heard throughout systole are referred to as holosystolic or pansystolic. These murmurs are associated with mitral or tricuspid regurgitation or with a ventricular septal defect. Mitral regurgitation is the most common of these and can be caused by rheumatic heart disease, mitral valve prolapse, calcification, or most commonly, left ventricular failure. S3 with gallop rhythm is indicative of left ventricular failure. This murmur is loud, high pitched, and blowing, and is heard best at the apex, radiating to the left axilla [38].
Tricuspid regurgitation is most often due to right ventricular failure, which may be secondary to pulmonary hypertension, left heart failure, mitral stenosis, or cor pulmonale. Additionally, rheumatic fever may have a direct effect on the tricuspid valve. A tricuspid regurgitant murmur is high pitched, is heard best at the lower left external border, and is associated with a parasternal lift [37,38].
Diastolic murmurs, although less common than systolic murmurs, are almost always associated with heart disease. Murmurs during diastole are caused by mitral or tricuspid stenosis or by aortic or pulmonary regurgitation. Mitral stenosis and aortic regurgitation are the most common of these murmurs. Mitral stenosis usually results from rheumatic fever, but calcification can also cause narrowing. The murmur occurs in middle-to-late diastole and has a characteristic "rumble" sound that is long and low pitched. It is best heard at the apex with the patient in the left lateral decubitus position. There is little radiation of this murmur, unlike the murmur of mitral regurgitation. Mitral stenosis is also associated with an opening snap and a diastolic thrill in the mitral area. Chronic mitral stenosis usually causes left atrial enlargement and hypertrophy and subsequent right ventricular hypertrophy or failure [38].
Tricuspid stenosis is similar to mitral stenosis in both timing and quality and is typically a result of rheumatic fever. This murmur is heard best at the lower left sternal border with the patient in a recumbent position.
Aortic regurgitation is an early diastolic murmur. It is high pitched and blowing and is heard best at the second right intercostal space and at Erb's point with the patient seated and leaning forward. As with other valvular diseases, aortic regurgitation is most often a result of rheumatic fever. However, other conditions, such as endocarditis, hypertension, and arteriosclerosis, may also cause this murmur [38].
Marked pulmonary hypertension may cause pulmonary valve regurgitation, another diastolic murmur, best heard at the second and third left intercostal space. The sound is medium to high pitched, rough, and brief [38].
The assessment of the patient with suspected cardiovascular dysfunction requires the examination of blood and urine for abnormalities. These tests are performed to establish a diagnosis, obtain general screening information, assess risk factors, and detect concurrent disease. The most common tests are serum lipids, serum cardiac biomarkers, serum glucose, coagulation studies, complete blood count (CBC), electrolytes, urinalysis, arterial blood gases (ABGs), chest x-ray, ECG, ambulatory ECG (Holter monitoring), echocardiogram, exercise stress testing, phonocardiogram, cardiac fluoroscopy, nuclear cardiology, cardiac catheterization, digital subtraction angiography (DSA), and hemodynamic monitoring.
The association between serum lipid (i.e., cholesterol, triglyceride, and lipoprotein) levels and CHD has been studied extensively. An elevated serum cholesterol level is directly related to the development of CHD—the higher the cholesterol level, the greater the risk. However, the inverse (lower serum lipid level, lower risk) is not true. Whether an elevated serum triglyceride level is a coronary risk factor is unclear. Some studies have documented an association between CHD and elevated triglyceride levels, but this association appears to be less predicative than the association of elevated total cholesterol and CHD [39].
Cholesterol and triglycerides are bound to plasma proteins and transported in the blood as lipoproteins. The major lipoproteins are classified as chylomicrons, very-low-density lipoproteins (VLDL), LDL, and HDL. The densities of lipoproteins vary according to the proportion of protein to fat and correspond to the protein portion of the lipoprotein. Each of the lipoproteins contains a different proportion of cholesterol, triglyceride, protein, and phospholipids. Chylomicrons and VLDL are composed primarily of triglyceride. LDL is predominantly cholesterol, while intermediate-density lipoprotein contains a combination of triglycerides and cholesterol. HDL is composed mainly of protein but also has 20% cholesterol [22].
Elevated LDL levels are positively correlated with atherosclerosis and an increased risk for developing vascular disease. Elevated HDL is negatively correlated with atherosclerosis, and studies demonstrate that elevated HDL levels appear to prevent plaque formation by converting cholesterol to a less active form. Total serum cholesterol measurements do not distinguish between LDL and HDL; lipoprotein electrophoresis provides this information [22]. Treatment plans are generally based on the ratio of total cholesterol to HDL.
Cardiac biomarker studies are performed primarily to document acute myocardial damage. Markedly elevated cardiac troponin or enzyme levels indicate the extent of the infarct. The enzyme levels most commonly obtained are creatine kinase (CK, also referred to as creatinine phosphokinase or CPK), CK isoenzymes, lactic dehydrogenate (LDH), LDH isoenzymes, and (less commonly) aspartame aminotransferase (AST) [40]. However, the practice of assessing cardiac enzymes (specifically CK) have been largely replaced by cardiac-specific troponin because of the latter's high concentration in myocardium, near-absolute specificity for myocardial tissue, absence in the blood of healthy individuals, and high clinical sensitivity.
Enzymes are catalytic proteins that accelerate biochemical reactions within cells. Each tissue of the body contains specific enzymes in varying concentrations. Under normal conditions, the intracellular concentration of enzymes is high, and the extracellular or serum concentration is low. Serum enzyme concentrations increase when cell damage occurs and the enzyme leaks into the blood. Thus, cardiac muscle damage can be detected by measuring serum levels of enzymes specific to the heart. The severity of the damage can be assessed by the amount and duration of the elevation [40,41]. However, in certain patients with less specific ST segment changes, cardiac enzymes may not be elevated, even in the presence of damage. Because cardiac troponins are more sensitive and specific, they have become the preferred serum cardiac biomarkers to assess in patients with suspected MI.
Cardiac Troponins
Measurement of troponin levels is crucial for identifying patients who have had an MI, but a diagnosis of MI should not be made on the basis of a single elevated troponin level, as elevated levels may be associated with other cardiac conditions, including tachyarrhythmia, high or low blood pressure, cardiac trauma, heart failure, myocarditis, and pericarditis. The time to initial elevation of cardiac troponin levels is 2 to 12 hours, with peak elevation at 24 hours (troponin I) and 12 to 48 hours (troponin T) [42,43]. Levels may remain elevated for 5 to 10 days (troponin I) or up to 14 days (troponin T) after an MI [43]. Although the greatest advantage of troponin over earlier biomarkers is their high sensitivity and specificity, their sensitivity is low during the first six hours after the onset of symptoms [43]. For most patients, in whom acute coronary syndrome is suspected, an MI can be ruled out or confirmed within six hours because of the high rate of delayed presentation associated with chest pain [42].
Creatine Kinase
Found in high concentrations in both skeletal and heart muscle, CK is the most useful enzyme measurement in the early diagnosis of MI. When the myocardium is injured, the serum CK level rises 3 to 6 hours after the event, peaks at 24 hours, and usually returns to normal within 72 to 96 hours. The specificity of CK activity is increased with the identification of the three CK isoenzymes: CK-BB, found primarily in nervous and smooth muscle tissue; CK-MM, found mainly in skeletal muscle; and CK-MB, which has the highest concentration in the cardiac muscle and is specific to the heart. Elevation in CK-MB is diagnostic of MI and appears within 4 to 6 hours after the onset of chest pain, peaks within 24 hours, and returns to normal within 48 to 72 hours. If the patient is not hospitalized within 24 hours from the onset of chest pain, the rise and peak of CK-MB may not be detected. CK-MM also increases with MI and persists for approximately five days. Serial documentation of CK and CK isoenzyme measurements provides a continuous assessment of myocardial necrosis [40,41].
Lactic Dehydrogenase
LDH is found in most tissues of the body, but the highest concentrations are in the heart, liver, brain, skeletal muscle, kidneys, and red blood cells. The specificity of LDH is improved when the LDH isoenzymes (LDH1 through LDH5) are measured. LDH1 and LDH2 are found primarily in the heart, red blood cells, and brain. Normally, LDH2 is proportionately higher than LDH1, but after an MI, the ratio is reversed and LDH1 is higher. This "flipped ratio" increases specificity in diagnosing MI. The LDH level is usually elevated within 8 to 12 hours after an MI, peaks within 24 to 28 hours, and remains elevated for 10 to 14 days. Because the LDH level remains elevated much longer than CK, it provides a significant diagnostic benefit in documenting MIs that occur more than 35 hours before testing [40,41,44].
Aspartame Aminotransferase
AST, previously referred to as serum glutamic oxaloacetic transaminase, was one of the original enzymes used in documenting damage to cardiac muscle, liver, pancreas, kidneys, brain, testes, and spleen. AST isoenzymes have not been identified, so their activity remains relatively nonspecific for myocardial damage. Therefore, it is rarely used to diagnose MI.
Elevated serum glucose and persistently abnormal glucose tolerance tests are associated with an increased risk of developing CHD. Although uncontrolled diabetes is recognized as a precursor of vascular disease, the exact physiologic mechanism remains unclear. The relationships among hyperglycemia, hyperlipidemia, obesity, platelet aggregation, and coagulation abnormalities are being investigated. Also being studied is the effect of certain oral hypoglycemic agents and insulin regimens on the vascular changes in diabetes. Transient hyperglycemia is found during periods of emotional and physiologic stress and is a common finding in patients with MI [40].
The major purposes of blood coagulation studies are to monitor the effectiveness of anticoagulant therapy and to detect deficiencies in serum clotting factors. Anticoagulant therapy with heparin is evaluated by obtaining the partial thromboplastin time (PTT). Prothrombin time (PT) is used to monitor the effectiveness of therapy with the anticoagulant warfarin. The therapeutic PTT range for heparin is 1.5 to 2.5 times the normal value; the therapeutic PT range for warfarin is 2 to 2.5 times the normal value. Oral anticoagulants take 3 to 5 days to reach therapeutic levels; therefore, heparin and warfarin are given in combination until the PT is prolonged [39].
Anticoagulant therapy may be used in patients with acute MI complicated by CHF to prevent clot formation. Patients who receive prosthetic valves often require chronic warfarin therapy to prevent clot formation on the valve. Certain medications may interact with the anticoagulants and potentiate or inhibit the effect. For example, quinidine, salicylates, and adrenocorticosteroids increase PT; antihistamines, barbiturates, and chloral hydrate decrease PT. Pharmacologic resources should be consulted, and a complete medication history should be obtained and reviewed when anticoagulant therapy is used [39].
The CBC includes a red blood cell count, corpuscular indices, white blood cell count, differential white cell count, hemoglobin, and hematocrit. The detection of anemia is important in patients with heart disease. Because it decreases the oxygen-carrying ability of the blood, anemia can aggravate CHF and precipitate chest pain. Anemia may also be secondary to hemolysis caused by a prosthetic heart valve. The white blood cell level is often elevated in patients with infections or injuries of the heart. The differential count, which includes neutrophils and lymphocyte counts, is also useful in assessing patients' cardiovascular condition, as neutrophils increase with bacterial endocarditis and MI and lymphocytes decrease with heart failure [39].
Cardiac function depends in part on the body's electrolyte balance. Myocardial muscle requires adequate stores of calcium to maintain the force of contraction, while potassium is needed to facilitate the myocardial cell's response to stimuli. The most common electrolyte abnormality in patients with cardiovascular disease is potassium imbalance. Depletion of potassium can result from vomiting, diarrhea, diuretics, insufficient intake, cardiac bypass surgery, and/or renal disease. A high serum potassium level is usually the result of renal failure, but it may also be caused by excessive potassium intake. Hypokalemia predisposes patients to dysrhythmias, particularly PVCs and ventricular fibrillation, and contributes to digitalis toxicity [39].
Patients with CHF or an acute MI are susceptible to electrolyte imbalance. These patients often complain of weakness, fatigue, and occasionally, muscle cramps. Serum electrolyte levels and ECG changes should be routinely monitored [39].
The retention of fluid by the kidneys is a compensatory response in acute heart failure and results in decreased output of urine. The urine that is output has a high specific gravity.
Nocturia is a frequent symptom of patients with CHF, and the nighttime urinary volume is often twice the daytime volume. Red blood cells may be present in the urine when the patient is receiving anticoagulant therapy. Urinary myoglobin is often present in patients with acute MI; however, it is not specific to the diagnosis [13].
ABG values, typically the acid-base balance (pH) and partial pressure of oxygen (PO2), are usually monitored when a patient is acutely ill. Although ABGs are not obtained routinely in cardiovascular assessment, they are useful for detecting the acid-base imbalances and hypoxemia often found in unstable cardiac patients. Uncompensated CHF may result in hypoxemia and either respiratory alkalosis or acidosis. Cardiogenic shock may cause hypoxemias and metabolic acidosis. Patients with acute MI tend to hyperventilate, which can lead to respiratory alkalosis and hypoxemia. Large doses of morphine for MI pain may also depress respirations and cause respiratory acidosis [39].
Significant information in the diagnosis of cardiac disease can be obtained through examination of the chest x-ray. The silhouette of the heart, cardiac chambers, and great vessels is observed, and the size and contour of the heart can be measured. Assessments are made by comparing the radiographic densities of the heart to those of surrounding structures, such as the lungs. In addition to cardiac enlargement, calcifications in the coronary arteries and great vessels can be seen. Pulmonary involvement from CHF and other cardiac dysfunction may be detected by x-ray examination as well. Pulmonary venous congestion and dilation of the pulmonary veins and artery may be observed [39].
A cardiac series enhances the routine chest x-ray. The contrast in the x-ray film is increased by administering barium to the patient. When the esophagus is filled with barium, it becomes more opaque. Chamber enlargement or aortic dilation can be detected by noting the displacement of the esophagus [39].
The ECG graphically represents the electrical activity of the heart. Cellular activity of the cardiac muscle generates electrical impulses that flow through the heart, causing cardiac contraction and relaxation. The flow of electrical impulses that leads to contraction is called depolarization. The straight line after the T wave of one pattern and before the P wave of the next pattern corresponds to the resting state. Electrical recovery of the heart muscle is referred to as repolarization. This electrical activity of the heart muscle can be measured by a system of electrodes placed at specific points on the body surface. Only electrical activity generated by the atria and ventricles can be recorded by the body-surface ECG; the specialized conducing tissues within the heart do not provide enough voltage to be detected by body-surface electrodes. The ECG displays the electrical activity as waveforms. As the electrical impulses travel through the atria, depolarization of the atria occurs. The ECG displays this activity as a P wave. The impulses continue through the heart to the ventricles. Ventricular depolarization is represented by a three-wave complex called a QRS complex. Because there are more muscle cells in the ventricles, ventricular repolarization can be measured on ECG and is recorded as a T wave [45].
For the standard ECG, electrodes are placed on each limb and on six sites across the precordium. The basic 12-lead ECG system is divided into bipolar leads, which have a positive and negative pole, and unipolar leads, which have only a positive pole. Waves are inscribed on the graph with a positive (upward) deflection when the direction of depolarization is moving toward the positive pole and negative (downward) deflection when depolarization is receding. The wave of depolarization in a normal heart flows from the base to the apex, slightly right, then left. Positive electrodes placed inferiorly to the apex and across the left precordium generally record positive deflections [45].
Three categories of leads compose the 12-lead ECG: the bipolar limb leads (leads I, II, and III); the unipolar augmented limb leads (aVr, aVl; and aVf); and the unipolar precordial leads (V1 to V6). Each lead or combination of electrodes records the electrical activity of the entire cardiac cycle from slightly different perspectives. The bipolar limb leads and the unipolar augmented limb leads measure the electrical potentials in the frontal plane, while the precordial leads measure the electrical potentials in the horizontal plane [45].
The bipolar limb leads measure the difference in electrical activity between two selected limbs [45]:
Lead I: Difference between the left arm and the right arm
Lead II: Difference between the left leg and the right arm
Lead III: Difference between the left arm and the left leg
The unipolar augmented limb leads are measurements of the electrical potential of each limb rather than the difference between two limbs. The height of the waveforms produced by these leads is small and therefore requires augmentation for easier reading. The ECG automatically augments the waves by increasing the size by 50%. The sites of the three augmented limbs leads are [45]:
aVr: Right arm
aVl: Left arm
aVf: Left leg (or foot)
The precordial leads are unipolar leads that record the electrical potential of each site in the horizontal plane. Six sequential positions across the anterior chest are used [45]:
V1: Fourth intercostal space at the right sternal border
V2: Fourth intercostal space at the left sternal border
V3: Midway between V2 and V4
V4: Fifth intercostal space at the midclavicular line
V5: Fifth intercostal space at the anterior axillary line
V6: Fifth intercostal space at the midaxillary line
The ECG recording is a valuable aid in cardiovascular assessment. For each pulse to be palpable with the QRS complex, ventricular contraction had to occur. Not only does it illustrate the rate and rhythm of the heart and conduction through the heart, an ECG also indicates enlargement of the atrial and ventricular chambers, inflammation of the pericardium, electrolyte (potassium) disturbance, or damage to the myocardium. A depressed T wave indicates a low serum potassium level. Any influence on the heart's ability to contract and relax can be documented by the ECG. In a normal physical exam, the 12-lead ECG reading is obtained for baseline information. To monitor effectiveness of cardiac drugs, serial ECGs may be performed [45].
The standard 12-lead ECG provides only a one-minute recording of the heart's rhythm; irregularities of the cardiac cycle that require longer recordings may not be detected. A rhythm strip, a recording of one to three selected leads, may be obtained for a longer reading [45].
Another limitation is that a normal ECG may be recorded even with significant CHD. The ECG can be combined with other tests (e.g., Holter monitoring) to increase diagnostic ability. In any clinical setting, ECG interpretations should be made in conjunction with the patient's history and physical exam.
Nursing Implications
The ECG is a noninvasive test that can be performed by a trained individual in any clinical setting. Nurses should prepare patients for the test by informing them about the purpose, the procedure to be used, and the risks involved. Explain to patients that the ECG provides a graphic description of the heart rhythm, which yields information about their cardiac status and the effectiveness of treatment strategies.
The ECG is performed with the patient in a supine position and his or her chest exposed for placement of electrodes. If hair is present, the electrode sites may be shaved. Adhesive electrodes are prepackaged with conducting gel. Ten electrodes are applied to the standard sites and attached to lead wires that connect to the ECG machine. A 12-lead ECG reading is obtained by selecting the indicators and completed in less than one minute.
The patient does not need to follow dietary restrictions prior to the procedure, but occasionally certain medications are discontinued before testing. The risks are minimal. A rare electrical hazard exists if proper electrical safety precautions are not followed. Written consent is not necessary, but the patient should give verbal consent.
Heart rhythm during daily activities can be evaluated by applying a portable ECG recorder (Holter monitor) that the patient wears for a specific length of time, usually 24 hours. The ECG is recorded, and the patient documents daily activities as well as any symptoms occurring with the activity in a diary. After completion of the test period, the data are translated into a standard graphic ECG reading by an electrocardioscanner. The ECG is reviewed, and the patient's documentation is correlated with the interpretation. This technique of ambulatory ECG recording is often referred to as Holter monitoring.
Ambulatory ECG monitoring is used most often with patients whose symptoms include episodes of dizziness, chest pain, fainting, or palpitations. This long-term monitoring permits a better correlation between the patient's subjective information and objective findings. Patients who have had open heart surgery or an MI can be monitored for 24 hours prior to discharge to detect latent dysrhythmias of abnormal hemodynamic responses. The effects of therapy may also be evaluated with this test [17].
Event monitors are similar to the Holter monitor in that they are portable and used to analyze the heart's activity. However, event monitors do not record continuously. They are generally activated by the wearer, although some will automatically begin recording if an abnormal heart rhythm is detected. There are two main types of event monitors: symptom event monitors and memory looping monitors; both require wearer activation. The symptom event monitor begins recording information from the heart's electrical signal upon activation and for the next few minutes. The memory looping monitor does this as well but also records information from a few minutes before the device is activated, allowing capture of symptom data from before, during, and after activation [46]. A wide variety of wireless cardiac monitoring devices (e.g., real-time smart phone monitoring, ECG patch monitoring, miniature injectable loop recorders) also are available and many others are under development [47]. Many of these devices are able to capture and transmit data directly to the patient's healthcare provider and have been found to have greater event detection rates than conventional ambulatory monitoring devices [48,49,50,51,52,53].
Nursing Implications
Ambulatory monitoring is a noninvasive test similar to a standard ECG. The nurse should provide instructions to the patient regarding the patient's involvement in the procedure. Three to five electrodes are applied only to the chest; not applying electrodes to the extremities provides the patient freedom to move. The electrodes are connected to lead wires of a portable ECG recorder.
The diary forms provided to patients should include headings for time, type of activity, and symptoms. The patient should be instructed to note the time and to record activities of daily living, including eating, walking, watching television, and sleeping, and to note any associated symptoms (e.g., dizziness, chest pain, fainting, palpitations). The patient may perform the usual activities but should avoid operating heavy machinery, microwave ovens, or electric shavers as well as bathing or showering, as these activities may interfere with the electrical signals of the ECG recorder. The patient may also be shown how to replace the electrodes if they are accidentally removed.
There are no inherent risks in ambulatory ECG monitoring. However, the patient may suffer skin irritation from the long-term use of electrodes taped to the body. Verbal consent should be obtained.
The American Association of Critical Care Nurses updated its practice alerts related to cardiac monitoring in 2017 [54].
The motion and dimensions of the cardiac structures can be measured by using high-frequency sound waves (ultrasound) and graphically recording the reflected sound (echo). This technique is called echocardiography. A sound-emitting transducer is applied to the patient's chest and focused on the heart. As the sound transects the various structures, echoes are produced and recorded. There are two methods for obtaining information: the M-mode and the two-dimensional (2D) mode [55].
The M-mode, the original technique, uses a narrow beam of sound and is described as giving an "ice-pick view" of the heart. The 2D mode, also referred to as the cross-section mode, transmits a wider sound beam. Thus, the images produced by the 2D have both motion and shape, whereas the M-mode images have only motion. The resulting images, along with those of the ECG, are usually recorded. The recording can be reviewed, and cardiac measurements that require comparison of several images can be obtained. Chamber size, ejection fraction (the percentage of left ventricular end-diastolic volume pumped), and flow gradient across the valves are some routine measurements [55].
The echocardiogram, frequently used in clinical cardiology, is a valuable tool in documenting valvular and congenital heart disease. In addition, the recording of cardiac movement permits objective assessment of cardiomyopathies, pericardial effusion, and ventricular aneurysm.
Nursing Implications
Echocardiography is a noninvasive test that can be performed in any clinical setting with an ultrasound machine. Nurses should explain to the patient that the echocardiogram will provide a recording of the movement of the heart valves and ventricular walls, which can be used to measure the functional ability of the heart.
The patient is placed in the recumbent position, and a transducer, lubricated with a gel to facilitate movement and conduction, is applied to the patient's anterior chest. As the transducer moves over the chest, images of the cardiac structure in motion are viewed. A second position is also used with the patient lying on the left side for better cardiac visualization. The images and the ECG are recorded. The entire procedure takes about one hour.
Ultrasonography has no significant risks. Verbal consent should be obtained; written consent is not necessary. However, echocardiogram has some limitations. The procedure for obtaining good images is difficult, particularly in patients who have a large, thick chest or a small heart [55].
During an exercise stress test, the ECG is recorded during prescribed exercise. A motorized treadmill or a bicycle ergometer provides the method of exercise, and the workload is gradually increased to a prescribed level. The objective of the test is to document the level of stress necessary to produce symptoms or signs. During the exercise, the ECG is monitored for ST-segment depression, ventricular dysrhythmias, conduction defects, and/or several heart-rate changes. Alterations in blood pressure are also monitored. The patient is observed for symptoms of fatigue, chest pain, or shortness of breath [33].
The exercise stress test is noninvasive and is used primarily in the prognosis and diagnosis of CHD. The results of the test are best used to document risk factors rather than as confirmation of diagnosis.
For patients who cannot engage in exercise sufficient to produce the amount of stress necessary, pharmacologic stress testing may be used. With this approach, cardiovascular stress induced by the administration of a drug, most commonly adenosine, dipyridamole, or regadenoson [56].
General indications for stress testing include:
Evaluation of dysrhythmias provoked by exercise
Differentiation in the diagnosis of chest pain
Identification of probable causes of atypical angina
Assessment of functional physical capacity and exercise levels in cardiac rehabilitation
Evaluation of therapeutic interventions
Unlike the resting 12-lead and ambulatory ECG, the exercise test has several contraindications, including any unstable cardiac condition (e.g., unstable angina), uncontrolled dysrhythmias, acute MI, uncompensated CHF, and severe hypertension. In general, the test is contraindicated for any patient who has a condition that could be aggravated by vigorous exercise. The patient should be interviewed and examined thoroughly before testing is initiated [33].
Limitations are to be considered when using an exercise test. The correlations between positive (abnormal) exercise tests and the presence of CHD have been poor. There is a high degree of false-positive and false-negative results in the diagnosis of ischemic heart disease. When the exercise test is performed in conjunction with myocardial perfusion studies, correlation with CHD increases [33].
Nursing Implications
Exercise stress testing is noninvasive, but because risks are associated with exercising until cardiovascular symptoms appear, the patient should be thoroughly informed and a written consent obtained. The test is performed with a cardiologist or other physician present, and the safety of the procedure depends primarily on adherence to the contraindications, termination of the exercise when the patient exhibits untoward symptoms and signs, and availability of personnel and equipment to manage complications.
The patient's understanding of the procedure and cooperation during the test are essential to successful exercise stress testing. To be adequately prepared, the patient should know the purpose, the procedure, and the risks involved. The purpose of the test is to evaluate the effect of activity on the heart as exercise on a moving treadmill is performed. Heart rhythm, blood pressure, and the patient's symptoms and signs are continuously monitored.
The patient should either fast overnight or have only a light meal no later than two hours before testing. Certain cardiac medications (e.g., digitalis, propranolol) may be discontinued before testing. The test is performed on a motorized treadmill or bicycle ergometer, so the patient should wear loose-fitting clothes and supportive shoes. A cardiovascular history and physical exam are obtained along with a resting 12-lead ECG, which is examined for any abnormalities (e.g., evidence of ischemia or serious dysrhythmias) that would preclude exercise stress testing. Several protocols for exercise stress testing have been developed, and depending on the patient's characteristics and physician preference, one protocol is chosen. The Bruce protocol for the treadmill, the most common, consists of several stages of increasing speed and slope, with each stage lasting three minutes. With this protocol, the maximum speed is 6 mph with an incline of 22% [17].
After the patient is instructed to report any untoward symptoms and is shown how to walk on the treadmill, the exercise test is started. The patient exercises until one of the following occurs: a predetermined heart rate is reached and maintained; symptoms such as fatigue, chest pain, or dysrhythmias appear; or significant ST-segment depression occurs [17].
In the postexercise period, the ECG and blood pressure continue to be monitored until the patient is completely recovered. The test is usually completed in approximately 20 minutes. The risk of an exercise test is small if contraindications and safety precautions are understood. A patient with pre-existing cardiovascular disease has the highest risk [17].
Phonocardiography, the graphic recording of heart sounds heard during auscultation, is an extension of the physical exam. The heart sounds are translated into electrical impulses, amplified, and recorded by a microphone. Each component of the heart sound and murmurs can be documented. External pulse recording of either the carotid artery, jugular vein, or apical pulse is taken at the same time as the ECG, which provides a timing reference for the sound [39].
The patient is placed in the normal auscultatory position and a pressure-sensitive transducer is applied to the selected pulse; the ECG reading is usually obtained through the standard limb leads. A contact microphone, used in the same manner as a stethoscope, is applied to the various auscultatory points. A phonocardiogram machine connects the three extensions and graphically records the pulse wave, ECG, and hearts sounds simultaneously [39].
Nursing Implications
The phonocardiogram is most useful in documenting heart sounds and murmurs that are almost inaudible with a stethoscope. The phonocardiogram's multiple-sound recordings, correlated with the ECG-timed pulse measurements, facilitate interpretation of the sounds. Phonocardiography is also helpful in teaching auscultation of heart sounds to students. Although the test is an extension of auscultation, the patient should be informed of the procedure's purpose and risk, which are the same as an ECG.
The movements of cardiac structures, the lungs, and coronary vessels may be viewed by fluoroscopy, permitting assessment of the degree of dysfunction of calcified heart valves and coronary vessels. In the past, the radiation exposure from this procedure was high, but now, with the use of image intensifiers and computers, the hazard is greatly reduced. The quality of the images has also improved [13].
Cardiac fluoroscopy is of less diagnostic value than the more sophisticated procedures now available, but it carries fewer risks and therefore may be used as a screening tool. Fluoroscopy is still used for guiding the placement and positioning of temporary pacemakers and intracardiac catheters [13].
Nursing Implications
Although fluoroscopy procedures are essentially noninvasive, the patient should be informed about the radiation exposure. No prior preparation is necessary unless barium is used. These procedures should be avoided during pregnancy.
Using radioactive tracer substances, cardiovascular abnormalities can be viewed, recorded, and evaluated. The use of radionuclide techniques in cardiovascular assessment is referred to as nuclear cardiology. Radionuclides are unstable isotopes that emit gamma rays as they decay to a stable form. The gamma rays are detected by a scintillation camera and converted to electrical impulses, which are then displayed and recorded [40,41].
The radionuclide techniques facilitate the evaluation of CHD, MI, ventricular wall function, valvular disease, and intracardiac shunts. The most commonly used radionuclides in nuclear cardiology are thallium (201Tl), technetium pyrophosphate (99mTc-PYP), and 99mTc-labelled red blood cells. Each radionuclide has different properties that contribute to the cardiovascular assessment. In general, two types of studies are performed in nuclear cardiology: the myocardial perfusion study and the cardiac function/performance study [41,44].
A myocardial perfusion study assesses the blood supply in the various regions of the myocardium, showing the effect of CHD. The most commonly used radionuclide in perfusion studies is 201Tl, a potassium analogue. Like potassium, thallium readily diffuses across the cell membrane and accumulates in myocardial cells, though its uptake depends on coronary blood flow and the ability of the myocardial cell membrane to extract thallium from the blood. In CHD or coronary spasm resulting in impaired coronary blood flow, the quantity of thallium reaching the myocardium is reduced and the uptake of thallium within the cells is low. If myocardial cell membranes are destroyed or nonfunctional, as in an MI, the thallium is not extracted from the blood into the cell, resulting in no thallium uptake [41,44].
The assessment of CHD has been greatly enhanced by combining exercise stress testing and thallium scintigraphy. With this approach, thallium is given intravenously during peak exercise and a scan is performed immediately after the test. This method permits detection of regions that may be perfused normally at rest but have reduced blood flow during exercise. A follow-up scan, performed about two hours after the first scan, assesses the redistribution of blood into the regions that were previously underperfused. Redistribution indicates that the myocardium is viable [41,44].
Thallium scintigraphy is also used to evaluate the effect of pharmacologic agents on coronary blood flow. Perfusion studies performed before or after coronary artery bypass assess the ability of the grafts to increase coronary blood flow. Thallium may be used to detect acute MI, but in order to be sensitive, the scan should be performed within six to eight hours after the onset of symptoms. Collateral blood flow interferes with the interpretation [41,44].
99mTc-PYP, which concentrates in zones of necrosis, is also used in imaging MIs. The mechanism of the uptake, although not yet clear, appears to be related to the calcium deposits in the infracted zones. Apparently, calcification may facilitate the extraction and concentration of pyrophosphate. Although pyrophosphate detects necrosis, uptake still depends on coronary blood flow. The infarct zone must have 30% to 40% normal blood flow for the radionuclide to reach the region. Abnormal scintigrams appear 10 to 12 hours after infarction. Any heart condition that results in myocardial cell damage (e.g., cardiac tumors, viral myocarditis, cardiomyopathy) may be assessed with 99mTc-PYP [41,44].
Ventricular function measurements, including ejection fraction and cardiac output, can be obtained through radionuclide angiography. The radionuclide used is sodium pertechnetate 99mTc, which attaches to red blood cells. Red blood cells labeled with 99mTc are large bodies that remain within the vascular system and provide for visualization of the vessels and cardiac chambers. Information from these studies is essentially the same as from contrast studies. However, radionuclide angiography is less invasive, requires less time, permits repeated studies, and has fewer inherent risks. Also, both right and left ventricular performance can be assessed at the same time [41,44].
The initial passage of the injected radionuclide is followed through the circulation with the scintillation camera, and images of the vessels, chambers, and volume waves are viewed. The study is completed within 30 seconds. The images are processed further by a computer, and a series of pictures is produced. The presence of intracardiac shunts is determined, and the ventricular ejection fraction and chamber volume are calculated. In clinical situations in which contrast studies are contraindicated, radionuclide angiography could be performed [41,44].
In gated blood pool studies, the radionuclide is injected and allowed to mix until an equilibrium of distribution is achieved. An ECG or echocardiogram is used to time the cardiac cycle. Dividing the cardiac cycle into time periods and recording measurements during these sequences is referred to as gating. Images are obtained at contraction (end-systole) and relaxation (end-diastole). With gated blood pool studies, measurements of ventricular ejection fraction, ventricular volumes, and ventricular wall motion may be obtained. The study can be performed at rest and during exercise [41,44].
Nursing Implications
Radioisotope cardiovascular assessment is relatively noninvasive, and the radiation exposure and risks are minimal. Because radioisotopes are used, there is no chemical toxicity. The studies do not require hospitalization or prior preparation. Nevertheless, the patient should be given information regarding the purpose of the study, and the procedure should be fully explained. A written consent should be obtained [17].
The most invasive but also the most definitive test in the diagnosis of cardiac disease is cardiac catheterization. With this procedure, a catheter is passed into the right or left side of the heart to obtain information on cardiac pressures, cardiac output, oxygenation, and the competency of intracardiac structures. When contrast dye is used to view the cardiac structures, the procedure is referred to as angiography, and this is usually combined with cardiac catheterizations. Risks include cardiac asystole or cardiac arrest [57,58].
Right-heart catheterization is performed by inserting a catheter through either the basilic vein, superior vena cava, femoral vein, or inferior vena cava. With continuous cardiac pressure monitoring and fluoroscopy, the catheter is advanced into the right side of the heart. Intracardiac pressure (i.e., right atrial, right ventricular, pulmonary artery, and pulmonary wedge pressures) are obtained. Blood samples are also withdrawn. Contrast dye is usually injected to detect any regurgitation from the pulmonary or tricuspid valves or to detect cardiac shunts [57].
The left-heart catheterization procedure is more invasive. The heart is approached either by passing the catheter from the right heart through the atrial septum, using a special needle to puncture the septum, or by retrograde insertion, which is performed by advancing the catheter from the brachial or femoral artery up the aorta, across the aortic valve, and into the left ventricle. This approach is used when coronary artery angiography is performed during the study. As with right-heart catheterization, pressure readings and blood samples are obtained. Mitral and aortic valve status and pressures in the left atrium, left ventricle, and aorta are evaluated. In addition, a ventriculogram is performed, and calculations are made of end-systolic volume, end-diastolic volume, stroke volume, and ejection fraction [57].
For coronary arteriography, the catheter is advanced into the aortic arch and positioned selectively into the right and left coronary arteries. Contrast dye is injected, and cineangiographic films are taken. Observing the flow of the dye through the coronary arteries provides information about the site and severity of coronary lesions. Drugs such as ergonovine and nitroglycerin may be administered during the study to evaluate the cardiovascular response [57,58].
With the more sophisticated noninvasive tests available, the need for cardiac catheterization has decreased slightly. Nevertheless, when either valvular or vascular cardiac surgery is indicated, a cardiac catheterization is performed to confirm the diagnosis and evaluate the surgical approach.
There are no absolute contraindications to cardiac catheterization. However, the patient must be physically able to tolerate the procedure and subsequent cardiac surgery if necessary. Evidence of acute MI, uncompensated CHF, or severe dysrhythmias may be contraindications. The risks and the benefits are evaluated before the procedure is performed [58].
Nursing Implications
Because cardiac catheterization is an invasive test, it is often frightening to the patient and the patient's psychosocial readiness should be assessed. Nursing assessment and teaching are essential for the patient to have a positive experience.
The purpose of the procedure should be explained fully. The nurse should relate that the major purpose for a cardiac catheterization procedure is to visualize the disease process in the coronary arteries and that the information obtained by this procedure is necessary to facilitate the patient's treatment. The patient will be required to recover for a few hours after the procedure. Standard preoperative tests (e.g., chest x-ray, ECG, CBC, urinalysis) are usually performed. The patient receives nothing by mouth after midnight or has only a liquid breakfast if the cardiac catheterization is scheduled for the afternoon. A mild sedative is usually given before the test. Nursing assessment includes the patient's vital signs, evaluation of peripheral pulses, and auscultation of heart and lung sounds. The site of catheter insertion is prepared, the area is shaved if necessary, and usually an antiseptic scrub is done [17].
The patient should be told how long the procedure usually takes, who will be present while it is taking place, and the layout of the physical environment. The patient will be kept in a supine position, which may become uncomfortable. The injection of the dye often causes a warm feeling throughout the body—often compared to "hot flashes"—that the patient may find disagreeable.
The most important nursing action following cardiac catheterization is assessing the groin for bleeding and the leg for color, warmth (circulation) and pulse. Postcatheterization care involves monitoring vital signs every 15 minutes for an hour, then every 30 minutes for an hour or until stable. Peripheral pulses and possible bleeding at catheter insertion sites should be assessed with the vital signs. An IV line for medication and fluid replacement is usually required. The patient is kept on bed rest (supine) for a few hours following the procedure [17].
The risks of cardiac catheterization, which are usually explained by the cardiologist, vary according to the patient's physical status and the procedures to be performed. Coronary arteriography, the most hazardous, may involve risk of death. Other risks include MI, cerebrovascular accident, arterial bleeding, arterial thromboembolism, and development of lethal dysrhythmias. Intracardiac trauma from the catheter may also occur. A written informed consent from the patient is necessary [17].
DSA is a newer approach to the traditional angiograms, requiring less patient observation during the procedure. Instead of injecting contrast dye directly into an artery, dye is injected into the venous system via the superior vena cava so it circulates through the heart and into the arterial system. A fluoroscopic image-intensifier displays the vessels and focuses (intensifies) the image. A computer then converts images into measurements. The first image obtained before the injection of the contrast dye is subtracted from the postinjection images. The image obtained from the computer subtraction is an enhanced image of the arterial system. Several vessels rather than one vessel can be evaluated with one injection of contrast dye. DSA is commonly used to diagnose abdominal aneurysms; thoracic aneurysms are more frequently diagnosed by magnetic resonance imaging (MRI) [59].
This technique has many advantages. It is less invasive than the conventional angiograms and requires less contrast dye, less time, and less radiation exposure, and it provides better images. It requires no hospitalization and can be performed on high-risk patients.
Intracardiac pressures can be measured and monitored continuously at the bedside in many critical care units. A balloon-tipped, flow-directed catheter is inserted percutaneously or by a venous cutdown into a large vein, such as the internal jugular or the subclavian, femoral, or brachial veins. The catheter (most commonly a Swan-Ganz catheter) is slowly advanced toward the right atrium. When it enters the right atrium, the balloon is inflated. Then, the flow of blood carries the catheter through the tricuspid valve, the right ventricle, and the pulmonary valve into the pulmonary artery. The balloon finally wedges into a branch of the pulmonary artery. The pulmonary artery wedge pressure (PAWP) is obtained, and the balloon is quickly deflated. The catheter floats back into the pulmonary artery and remains in this position for continuous monitoring. The pressures routinely monitored are the pulmonary artery systolic pressure (PASP) and diastolic pressure (PADP), PAWP, and cardiac output. The PASP represents the pressure generated by the contraction of the right ventricle, whereas the PADP represents the filling pressure of the right ventricle. These pressures are monitored to assess right-sided heart function and pulmonary resistance. In the absence of lung dysfunction and mitral valve stenosis, PAWP reflects left atrial pressure and left ventricular end-diastolic filling pressure. Thus, the function of the left heart can be assessed. The best hemodynamic indicator of left ventricular hypertrophy is the measurement of pulmonary capillary wedge pressure (PCWP) [37,38].
Catheters with thermistors can be used to measure cardiac output by the thermodilution technique. A bolus of a solution with a known temperature is injected into the proximal port of the catheter, and the thermistor at the distal end measures the temperature change of the diluted solution. A bedside cardiac output computer connected to the catheter records the data, calculates the temperature curve, and displays the cardiac output [38]. These intracardiac pressure readings are valuable aids in the assessment of the critically ill patient, and serial readings assist in evaluating the effectiveness of the therapeutic plan.
Nursing Implications
As an invasive procedure, hemodynamic monitoring carries several risks. The catheter site, and subsequently the blood, may become infected. Endocarditis may occur but can be prevented by using sterile technique and observing the patient for early signs of infection. Other serious complications include thromboembolism from blood clotting to the catheter; air embolism from balloon rupture, faulty line connections, or improper procedure; ventricular dysrhythmias due to ventricular irritation from the tip of the catheter floating back into the right ventricle; and pulmonary artery rupture from over distention of the balloon or from the tip of the catheter piercing through the artery. The patient should be prepared for the procedure and the continuous presence of the catheter, IV line, and monitors. Explain that the situation is temporary and the information obtained will assist in the overall treatment plan. The family should also be informed. A written consent is necessary, but often, because the situation is critical and the patient unconscious, obtaining a written consent from the patient may not be feasible. In these situations, the family may give a written consent [17].
CHD results from atherosclerosis and is the most commonly recognized cause of myocardial ischemia. CHD is defined as narrowing of the arteries causing a decreased lumen and decreased blood flow through the coronary arteries. The following factors speed the development of plaque, which in turn impedes blood flow to the myocardium, thereby decreasing oxygen availability to tissue [60]:
Elevated cholesterol and triglyceride levels
Elevated blood pressure
Infection that initiates the inflammatory response
Elevated iron levels
Elevated homocysteine levels (and comorbid vascular disease)
Injury to the lining of the artery occurs, resulting in increased permeability of the endothelial cells and allowing components of the plasma to enter. An inflammatory reaction occurs with the injury, bringing macrophages and platelets to the injured site. Hemorrhage into the plaque produces thrombi; thrombus formation within the lumen of the artery is initiated by platelet aggregation. Embolization of a thrombus or plaque fragment can occur. Cholesterol, fat, and thrombi can develop into a plaque formation. Progressive narrowing of the lumen by plaque enlargements results in ischemia (insufficient blood flow leading to decreased oxygen supply to the myocardium) due to narrowing of the artery lumen. ECG changes (e.g., inverted T wave, depressed ST segment) occur during an acute or severe episode of ischemia. Also, chest pain or angina pectoris occurs [2,60,61].
Cholesterol and triglycerides are circulated in the blood as lipoproteins. HDL, known as "good cholesterol," is protective against CHD by taking fats away for breakdown. LDL and VLDL help fat remain in cells and are known as "bad cholesterol." Signs and symptoms include chest pain, hypertension, tachycardia, tachypnea, pallor of the skin, and occasionally diminished peripheral pulses. The comparable symptom of atherosclerosis in the legs is intermittent claudication, which presents with cramping in the lower extremities, especially when walking or with exercise [2,60,61].
Patients with known or suspected CHD should be assessed for pain, vital signs, and appearance in addition to taking a complete history (Table 1). Priorities of care include patient teaching regarding medication management. Antihyperlipidemic agents and those that lower triglycerides include 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (e.g., atorvastatin [Lipitor], cerivastatin [Baycol]). In addition to these agents, patients may require antiplatelet therapy with acetylsalicylic acid (aspirin) and antianginal medication (nitroglycerin). Antihypertensives and/or antimicrobials may be added if indicated. Patients should be advised regarding risk factor management and secondary prevention. Recommend a low-fat diet, regular exercise, regular checkups, cessation of smoking, and stress management. Teach patients to recognize signs and symptoms of CHD, including shortness of breath, chest pain, and dyspnea on exertion, and to stop and rest at the onset of discomfort [62; 63].
NURSING ASSESSMENT OF CORONARY HEART DISEASE
| Assessment Components | Notes | |||||
|---|---|---|---|---|---|---|
| Pain |
| |||||
| Potentiating factors | Exercise, stress, cold temperature, anemia, substance abuse, thyrotoxicosis, hyperthyroidism | |||||
| Relieving factors | Rest, removal of stressors | |||||
| Diagnostic tests |
| |||||
| Other components |
|
Angina pectoris is defined as chest pain caused by myocardial ischemia from reduced blood flow, reduced oxygen supply for demand of myocardium, or a temporary or reversible cause. It is classified as stable, unstable, or Prinzmetal and is the most common symptom of CHD. The pain of angina may last from 30 seconds to 30 minutes. It has been described as a heaviness; a squeezing, viselike pain; or a crushing pain over or near the sternum. The pain may radiate into the left arm, neck, jaw, or back. Difficulty breathing may accompany the pain. Angina usually occurs with increased activity or exposure to a cold environment, when myocardial oxygen needs increase. Treatment may be medical, surgical, or a combination of the two. Causes include CHD, coronary spasms, thrombi, or any condition that creates an imbalance between oxygen supply and demand of myocardium [65].
When there is decreased blood flow to the myocardium and/or increased demand for oxygen that cannot be met, angina occurs. This is the body's way of communicating that there is not enough oxygenated blood—a forewarning that an MI may occur. Reduced oxygen supply causes a switch from aerobic metabolism to anaerobic metabolism, which can result in [65]:
Lactic acid build-up
Altered cell membrane permeability, releasing histamine, bradykinins, and specific enzymes that stimulate terminal nerve fibers in the myocardium to send pain impulses to the central nervous system
CHF, congenital heart defects, pulmonary hypertension, left ventricular hypertrophy, and cardiomyopathy, which also cause decreased oxygen supply to the myocardium
Silent ischemia (decreased oxygen supply without warning signs of pain)
Coronary artery spasm (temporary narrowing of coronary arteries and angina resulting from an unknown etiology)
Increased oxygen demand of the myocardium can be caused by anemia, exercise, thyrotoxicosis, substance abuse (e.g., cocaine use), hyperthyroidism, and emotional stress. As noted, the types of angina are stable, unstable, and Prinzmetal (variant) (Table 2) [65].
TYPES OF ANGINA
| Angina Type | Characteristics | |||||
|---|---|---|---|---|---|---|
| Stable |
| |||||
| Unstable |
| |||||
| Prinzmetal (variant) |
|
The main symptom of angina pectoris is pressure or heaviness in the chest, which the patient may describe in other terms (e.g., burning). This may be accompanied by sweating, light-headedness, hypotension, pulse changes (low or high), or indigestion. Pain may radiate to the arms, jaw, abdomen, or back. The ECG will show depressed ST segments [65].
Assessment of patients with suspected angina includes symptom analysis of the pain (e.g., location, duration, precipitating and relieving factors), vital signs, respiratory patterns, color (pallor), anxiety, and heart sounds. The patient should be prepared for diagnostic tests, including ECG, exercise or pharmacologic stress test (with and without contrast), radiographic study (angiogram), CK (with isoenzyme), LDH (with isoenzyme), and troponin, to rule out MI. Tests for gastroesophageal reflux disease may be ordered to rule out gastric causes [18,64].
Patients are usually administered an antianginal medication such as nitroglycerin. Antiplatelet agents and beta blockers may also be indicated. Calcium channel blockers are used to decrease afterload (i.e., pressure the heart must pump against) and spasms; analgesics may be prescribed for headache (a common side effect of nitroglycerin). Some patients may benefit from stool softeners, and class Ib antiarrhythmics may be given prophylactically if PVCs occur (though there is a risk of asystole with MI) [66,67].
The goal of treating angina is to reduce oxygen demand and improve blood and oxygen supply. Primary nursing care is related to education of the patient. Patients should receive information regarding the signs and symptoms of angina pectoris and the use of nitroglycerin. Typically, patients are advised to take one tablet every five minutes for 10 minutes after the onset of symptoms and to report if no relief is obtained. Nitroglycerin may also be used prior to activities that may cause angina (e.g., sexual activity, work-related activities). Oxygen should be administered as needed. Treatment may consist of percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft surgery to prevent an MI. The patient should be instructed about these procedures [18,64].
Monitor vital signs, heart sounds, and ECG pattern. The heart rate may increase as a response of the sympathetic nervous systems if blood pressure drops. Instruct the patient not to strain with bowel movements or create excess pressure by bending; administer stool softeners as needed. Help patients identify known stressors and begin a cardiac exercise program. Pulse should be monitored before beta blockers; these medications may be held if the pulse is less than 60 bpm. Blood pressure should be checked and recorded before each nitroglycerin dose, as this agent can cause dizziness, headache, and drastic hypotension due to relaxation of the smooth muscles and vasodilation. Morphine sulfate may be administered in small doses if the systolic blood pressure is less than 100 mm Hg. Blood pressure should also be carefully assessed during the administration of calcium channel blockers [18,64].
MI usually results from atherosclerotic CHD. It is defined as the death of cells in the myocardium due to prolonged or severe ischemia to that area. Blood clots form on or adjacent to the atherosclerotic lesions, and these clots cause an MI by contributing to the occlusion of the coronary arteries. Alternatively, some MIs are caused by sudden onset ventricular fibrillation. Other causes include embolus, thrombosis, atherosclerotic occlusion, and prolonged vasospasm. Signs and symptoms include pain, dyspnea, nausea and vomiting, anxiety, diaphoresis, pallor, dysrhythmias, and elevated temperature. Treatment includes relieving discomfort, preventing and treating complications, promoting healing of the damaged myocardium, and promoting rehabilitation.
Cellular injury occurs because of a lack of oxygen over time (ischemia); prolonged (20 to 45 minutes) ischemia can lead to necrosis. Coronary artery occlusion results in cell death distal to the occlusion. Scar replaces muscle, but scar tissue does not contract or conduct an impulse. Damage begins at the subendocardial layer and progresses to the epicardium within one to six hours [68,69].
Damaged cells cause a decrease in myocardial contractility, resulting in decreases in stroke volume, cardiac output, blood pressure, and tissue perfusion. Prolonged ischemia may lead to the development of collateral circulation (i.e., the outgrowth of tiny vessels), which provides additional pathways for delivery of oxygen and nutrients to the area of ischemia [68,69].
MIs are described by location and the affected myocardial surface (Table 3). These descriptions provide insight into the expected symptoms/signs and the severity of the infarction.
MYOCARDIAL INFARCTION DESCRIPTORS
| Affected Area | Description |
|---|---|
| Location | |
| Anterior | Usually occurring in the area supplied by left anterior descending coronary artery |
| Posterior | Usually occurring in the area supplied by the right coronary artery |
| Lateral | Occurring in the area supplied by the left circumflex artery |
| Myocardial Surface | |
| Transmural | Affecting the endocardium to epicardium |
| Subendocardial | Affecting the endocardium surface into the myocardial muscle |
| Intramural | Affecting patch areas of myocardium |
The pain associated with MI is typically felt as a tightness or crushing feeling in midsternal area, radiating to the jaw, arms, neck, abdomen, or shoulder. It usually lasts 15 to 20 minutes and is not relieved with nitroglycerin or bed rest [68,69]. Some events are pain-free; this is referred to as a silent MI.
Duration of pain is important in distinguishing MI from angina pectoris. The sudden onset of pain, usually not associated with activity, may awaken the patient in the middle of night. Tachycardia is the result of the increased need of oxygen for cardiac tissue. Diaphoresis, tachypnea, dyspnea, elevated temperature, change in level of consciousness, anxiety, feeling of impending doom, and nausea/vomiting may also occur. ECG changes include depressed or elevated ST segment (most common), inverted T wave, and/or formation of Q waves [68,69]. Arrhythmias, PVCs, and ventricular tachycardia may be present [69].
Enzymes are normally present in the muscle fiber of the body and are not elevated in the serum unless injury to the muscle occurs. When any muscle is injured, the enzymes are released into the blood stream. Isoenzymes are a breakdown of the total enzyme level, and when certain isoenzymes are elevated, this gives an indication of which particular muscle has been damaged [69]. As discussed, the specific CK isoenzyme will give insight into the muscle affected. When the total CK level is elevated, it is important to assess for an elevated CK-MB isoenzyme to determine if an MI has occurred. The same is true of assessing LDH isoenzymes. Specifically, an elevated LDH1 isoenzyme indicates an MI.
The most common initial reaction to an MI is to deny the severity of symptoms and delay health care. The nursing assessment of patients with suspected MI includes symptom analysis of chest pain (e.g., location, duration, radiation, quality), associated symptomatology (e.g., diaphoresis, nausea/vomiting), cardiac rhythm, and nonverbal cues of discomfort. Recommended diagnostic tests are [64]:
CK and LDH with isoenzymes
Troponin level
12-lead ECG
Chest x-ray
Angiogram
Echocardiogram
Stress tests
ABGs
Electrolytes
CBC
Pulse oximetry (for quick reference of oxygen saturation)
Prognosis related to the extent of heart damage is determined by the presence or absence of collateral circulation [68,69].
Most patients with MI are administered nitroglycerin for vasodilation, often in the form of sublingual/topical spray, nitroglycerin drip, and/or nitroglycerin paste. Analgesics are given as needed for pain relief. Morphine sulfate is indicated if the blood pressure is too low or pain is unrelieved by nitroglycerin. The scheduled dose of morphine should be held if the patient's respiratory rate is 10 breaths per minute or less. Other usual medications include stool softeners, calcium channel blockers, beta blockers, antihypertensives, and anticoagulants such as heparin, warfarin, or low-molecular-weight heparin (e.g., enoxaparin) [66,67]. Thrombolysis should be considered.
Antiarrhythmics may be given prophylactically. Options include [67]:
Amiodarone, procainamide, or lidocaine, for ventricular antidysrhythmics such as PVCs
Atropine, for bradydysrhythmias
Adenosine, for tachydysrhythmias
Monitor for major side effects of medications, including hypotension, bradycardia, bleeding, constipation, and depression.
Supplemental oxygen is given via nasal cannula at 2–5 L/minute. The head of the bed should be elevated for respiratory comfort, and IV access should be maintained. Monitor bowel movements, encouraging the patient not to strain. Electrolyte replacement should be done as necessary. It is important that patients have physical and psychological rest as well as emotional support. Reassess patients frequently for pain relief or continued pain using a scale to assess the quality of the pain. Patient/family teaching may be initiated when they are ready to listen and absorb the information [68,69].
Monitor and record vital signs with administration of nitrates, beta blockers, and calcium channel blockers. These medications should be held if the pulse is less than 60 bpm or systolic blood pressure is less than 100 mm Hg. The cardiac rehabilitation program will begin as soon as the patient is able to participate.
CHF usually begins in the left ventricular region of the heart and manifests as pulmonary congestion and edema. It is defined as the inability of the heart to pump sufficiently to meet the metabolic needs of the body, causing decreased tissue perfusion due to decreased cardiac output. Heart failure indicates that the heart is unable to adequately perform its function of pumping blood throughout the circulatory system [70]. Left heart failure and right heart failure describe the side of the heart that has the primary impairment. It is helpful to think of heart failure as left or right because the symptoms and signs may provide clues as to the location of the primary problem. Keep in mind, however, that failure of one side of the heart usually leads to failure of the other side. CHF may be acute (pulmonary edema or cardiogenic shock), chronic, or both [71].
Signs and symptoms depend on the severity of the failure and the side of the heart that is predominantly affected. They include [70]:
Difficult breathing
Coughing of blood-tinged, frothy sputum
Fatigue
Edema
Nocturia, oliguria
Weight gain
Cerebral symptoms (e.g., dizziness, syncope)
Gastrointestinal symptoms (e.g., anorexia, bloating)
Abdominal pain
The heart pumps blood to the pulmonary and systemic vascular systems in order to maintain an adequate oxygenated blood supply to the heart and produce sufficient cardiac output to meet the metabolic needs of the body. Damage to the heart, regardless of cause, leads to decreased cardiac output and decreased tissue perfusion. Two dysfunctions contribute to CHF: diastolic and systolic. Diastolic dysfunction occurs when the ventricle pumps against an extremely high afterload, as in primary hypertension. This effect leads to a decrease in the ventricle's ability to comply, decreased filling of ventricles (preload), and decreased stroke volume and hypotension. Systolic dysfunction occurs when the ventricle experiences damage, as in a MI, and the ventricle cannot contract effectively. With this condition, the stroke volume decreases, leading to an increased preload (volume of blood returning to the heart creating the "stretch" or tension of the myocardial fibers at end of diastole) and the ventricle becomes distended. As a result, signs of decreased cardiac output can manifest [71,72].
Compensatory cardiac mechanisms for heart failure include Frank-Starling mechanism, myocardial hypertrophy, and tachycardia. As noted, the Frank-Starling mechanism is the force of contraction determined by the amount of stretch. This mechanism is able to augment stroke volume.
Following myocardial injury, the inciting event in heart failure involves adaptation of the cardiac myocyte to increased wall stress in order to maintain an adequate cardiac output. The primary myocardial response includes myocyte hypertrophy and remodeling, usually of the eccentric type. Although the hypertrophic state is induced by mechanical distention, there is also humoral control of myocyte hypertrophy, including endothelin, angiotensin II, and norepinephrine.
Decreased cardiac output stimulates aortic baroreceptors, causing the release of norepinephrine to increase heart rate and contractility. This causes vasoconstriction, increasing blood return to the heart and cardiac output. Decreased cardiac output leads to decreased renal perfusion, which in turn leads to the release of renin from juxtaglomerular cells.
Activation of the renin-angiotensin-aldosterone system causes vasoconstriction, stimulates the adrenal cortex to produce aldosterone, and releases ADH. This inhibits water excretion in distal tubules, causing vasoconstriction and increasing blood pressure. Vasoconstriction initiates increased blood return to the heart (preload), causing greater stretch (Frank-Starling law) and increasing cardiac output. Atrial stretch stimulates release of atrial natriuretic factor (ANF) to balance the effects of renin and aldosterone, promote sodium and water secretion, and inhibit norepinephrine, renin, and ADH. Ventricular hypertrophy results from excess fluid volume and pressure causing cells to enlarge and stiffen, thereby decreasing the force of contraction. The compensatory mechanisms hasten the deterioration of cardiac function and the onset of heart failure. Tachycardia decreases diastolic filling time, compromises coronary artery perfusion, and increases cardiac output [71,72].
Over time, beta-receptors in the heart become less sensitive to sympathetic nervous system stimulation, thereby decreasing heart rate and contractility. Alpha receptors on peripheral blood vessels are more sensitive, which promotes vasoconstriction and increases afterload and cardiac workload. Chronic ventricular dilation leads to ventricular wall thinning, degeneration, and loss of effective contractility. Chronic atrial dilation leads to depletion of ANF, leaving renin, aldosterone, and norepinephrine to continue unabated. This causes increased preload, afterload, and further deterioration of heart failure. Ultimately, the oxygen supply to the tissue is impaired to the point of leading to further ischemia and tissue death (infarction) [71,72].
Left-sided heart failure is more common than right-side failure and results from left ventricular wall damage or dilatation. This results in left ventricular and atrial end-diastolic pressure increasing and cardiac output decreasing. Impaired left ventricular filling causes congestion and increased pulmonary vascular pressures [71,72]. Increased pulmonary vascular pressure induces a shift of fluid interstitially and leads to alveolar congestion and pulmonary edema. Increased pulmonary artery pressure is defined as a mean pressure greater than 15 mm Hg as measured by pulmonary artery catheter.
Signs and symptoms of left heart failure include [71,72]:
Fatigue
Activity intolerance
Dizziness
Syncope
Dyspnea (shortness of breath)
Cough
Orthopnea
Pulmonary crackles on auscultation
S3 heart sound
Tachycardia with possible atrial dysrhythmias
Decreased urine output
A popular trick for remembering the usual pulmonary manifestation of left-side heart failure is to recall that both of the words "left" and "lung" begin with the letter L.
Right-sided heart failure is caused by pulmonary hypertension (cor pulmonale), left-sided heart failure, or right ventricular infarction. Pulmonary hypertension causes increased pressure the right ventricle must pump against. As a result, the right ventricle cannot empty, leading to hypertrophy and dilation. Right ventricular distention leads to blood accumulating in the systemic venous system. The trick to remembering this correlation is the letter R, for "right" and "rest of the body" [71,72].
Increased venous pressure causes abdominal organ congestion and peripheral edema [72]. Lower extremity edema occurs in patients who are ambulatory, while bedridden patients will experience sacral edema. Liver engorgement will lead to right upper quadrant pain. Anorexia and nausea occur with gastrointestinal venous congestion.
Biventricular failure occurs when a patient experiences signs and symptoms of both left and right failure and jugular venous distention occurs. Peripheral edema is reabsorbed into circulation when the patient goes to bed and feet are elevated, causing fluid overload, pulmonary congestion, and paroxysmal nocturnal dyspnea. In addition, severe heart failure predisposes the patient to dyspnea at rest. S3 and S4 heart sounds may be heard [71,72].
Hepatomegaly and splenomegaly result from abdominal engorgement or congestion. Signs include increased abdominal pressure, gastrointestinal problems (e.g., anorexia, nausea, vomiting), decreased digestion, and impaired absorption of nutrients. Dysrhythmias may develop from myocardial distention causing interference with conduction and lowering cardiac output. Cardiogenic shock or acute pulmonary edema may develop as cardiac function deteriorates [71,72].
Nursing assessment includes vital signs, hemodynamic monitoring, heart rhythm, pulse quality (e.g., full, bounding, thready), respiratory status (e.g., dyspnea, shortness of breath, tachypnea), lung sounds (e.g., crackles, rhonchi, wheezing), heart sounds (e.g., S3 or S4, murmur or rub), jugular neck vein distention, abdominal assessment (e.g., palpation, distention, ascites), edema, and activity tolerance [73,74]. Diagnostic tests include:
ECG
Signs of ischemia: Depressed ST segment, inverted T wave
Signs of injury: Elevated ST segment, Q wave development
Electrolytes
Liver function test
ABG analysis
Chest x-ray
Echocardiogram
Pulse oximetry
Tests to rule out an MI may be done if the patient is also having chest pain [73,74].
The primary goal of the management of CHF is to reduce the oxygen demand of the myocardium. As such, the treatment plan consists of physical and psychological rest, prescribed medications to reduce cardiac workload and improve contractility, management of symptoms, and amelioration of decreased cardiac output.
Vital Signs
Decreased cardiac output stimulates the sympathetic nervous system to increase heart rate and diastolic blood pressure. As the heart's pumping action deteriorates, blood pressure decreases. Assessment of subtle changes in vital signs may lead to early intervention [18,19].
Auscultative Heart and Lung Sounds
S1 and S2 may be diminished as cardiac function fails. S3 (ventricular gallop) is an early sign of heart failure, and S4 (atrial gallop) may also be present. Basilar crackles may be heard. Increasing crackles and dyspnea mean worsening of condition and should be reported immediately when heard [19].
Level of Consciousness
Mental changes occur as blood flow to the brain decreases. Oxygen saturation changes may also lead to changes in the patient's level of consciousness [19].
Urine Output
Decreased blood flow to the kidney leads to decreased output. In addition to gauging the severity of the disease, assessing urine output assists in evaluating the effectiveness of some medications used to treat heart failure, such as diuretics and positive inotropic agents [19].
Notify the physician if output is equal to or less than 30 cc/hour. Use of diuretics reduces circulating volume and can produce hypovolemia even in the presence of peripheral edema. Decreased urinary output could indicate decreased cardiac output and renal ischemia [18].
Color and Temperature of Skin
Cool, clammy skin frequently presents in the acute phase of heart failure. Pale, cyanotic coloring is often seen in later stages as cardiac pumping action deteriorates [19].
Weight and Abdominal Circumference
Weight provides an objective measure of fluid status; 1 liter of fluid is equal to 2.2 pounds of body weight. The patient should be weighed daily [18].
Venous congestion also leads to development of ascites and may produce gastrointestinal complaints. Increasing abdominal girth is a sign of increased ascites production. Therefore, abdominal girth should be assessed, measured, and recorded at every shift [18].
Cardiac Dysrhythmias
Sinus tachycardia is usually present in patients with heart failure. Atrial dysrhythmias are also frequently seen, including atrial fibrillation, atrial flutter, premature atrial contraction, and atrial tachycardia. Ventricular dysrhythmias develop as ventricular hypertrophy and dilation occur [18].
Supplemental oxygen is given to decrease the effects of hypoxia and ischemia. The patient should be placed at rest with the head of bed elevated (to 45 degrees) to improve respiratory function and reduce cardiac workload [18]. It is usually necessary to assist the patient with personal care needs. A bedside commode may be used to reduce patient effort necessary in positioning on a bed pan. Any straining (e.g., Valsalva maneuver, isometric exercises) should be avoided.
Promote psychological rest and minimize anxiety to reduce oxygen consumption and improve cardiac function. This involves maintaining a quiet environment, allowing expression of fear, and explaining procedures to the patient's satisfaction. When possible, remain with the patient to reduce anxiety [18].
Fluid Overload
Fluid volume excess may be assessed by the patient's respiratory status. Declining respiratory status indicates worsening left heart failure. Shortness of breath, dyspnea, cough, orthopnea, and paroxysmal nocturnal dyspnea may be seen in these patients [18].
Acute pulmonary edema with air hunger and tachypnea can develop rapidly [18]. The patient begins to panic and may feel an overwhelming sense of impending doom. Patients will request to sit straight up in bed and will have a productive cough with large amounts of soft pink, frothy sputum.
Assess for signs of deteriorating condition, including the following hemodynamic data (if a pulmonary artery catheter is in place) [18]:
PCWP
Pulmonary artery pressure
Right atrial pressure
Systemic vascular resistance
Cardiac output
Cardiac index
Steps should be taken to decrease fluid overload. If ordered, fluids should be restricted. Minimal or no cardiac reserve for increased activity leads to self-care deficits. Allow adequate rest periods prior to activity, and assess for signs of decreasing activity tolerance. Assist with activities of daily living while also allowing the patient to do as much as possible to provide some sense of control and to reduce feelings of helplessness. Passive and active range of motion exercises may be provided to progressively promote improved cardiac function [18].
Patient teaching regarding discharge instructions should include information on [18]:
Low-sodium diet
Performing activities as independently as possible
Spacing meals and activities
Allowing time for rest and relaxation
Stopping any activity that causes chest pain or shortness of breath
Avoiding straining
Beginning a slowly progressive exercise program
Pharmacotherapy
A wide variety of medications is used to manage heart failure and its complications. Although both digitalis and low-dose angiotensin-enzyme converting (ACE) inhibitors enhance urinary sodium excretion, few volume-overloaded heart failure patients can maintain proper sodium balance without the use of diuretics such as furosemide (Lasix). ACE inhibitors should be given as the initial therapy in the absence of fluid retention. In patients with fluid retention, ACE inhibitors should be given together with diuretics. When given in concert with ACE inhibitors, beta blockers reverse the process of left ventricular remodeling, improve patient symptoms, prevent hospitalization, and prolong life. Angiotensin receptor blockers (ARBs) may be used in patients who are intolerant of ACE inhibitors, but they should be used with caution in patients with a history of angioedema with ACE inhibitors [75]. ARBs have been shown to reduce hospitalization and mortality [70]. The mechanism of action of ARBs is similar to that of ACE inhibitors, but ARBs do not cause the inhibition of kininase. ARBs inhibit the renin-angiotensin-aldosterone system by blocking the binding of angiotensin II to its receptor. This leads to vasoconstriction and prevents the release of aldosterone [70]. Two aldosterone receptor antagonists (spironolactone and eplerenone) are available in the United States and are recommended for select patients with heart failure [75]. Other medications used in the management of heart failure include positive inotropic medications to promote urinary output (e.g., dopamine), analgesics (e.g., morphine), antihypertensives, direct vasodilators, beta blockers, and antidysrhythmics [63].
Close monitoring and recording of drug therapy should be done. Monitor apical pulse for one minute prior to administering digoxin, and hold the medication if the pulse is less than 60 bpm. Monitor vital signs and intake and output carefully while administering dopamine [18]. Low doses may result in renal perfusion, and moderate-to-high doses are associated with elevated blood pressure. Avoid pushing IV furosemide too rapidly, as ototoxicity can occur [62].
Electrical stimulation of the normal heart causes depolarization of the myocardium, and this depolarization causes a synchronized, rhythmic contraction of the myocardium, producing a beat and propelling the blood through the arteries and capillaries. Cardiac dysrhythmias are defined as alterations in this stimulation that can affect the synchronized pattern of contractions and the efficiency of the heart. Normal sinus rhythm conduction progresses from the SA node to the AV node, the bundle of His, the bundle branches (right and left simultaneously), and finally to the Purkinje fibers, causing contraction of the atria and ventricles. Cardiac dysrhythmias are caused by many factors and may be benign or life-threatening. Any dysrhythmia can affect cardiac function, regardless of cause, and the patient's response to the rhythm determines urgency of treatment.
Dysrhythmias originate as sinus, atrial, junctional, ventricular, or heart blocks. Some more common dysrhythmias include [76,77]:
Sinus dysrhythmia
Sinus tachycardia
Sinus bradycardia
Premature atrial complexes
Atrial flutter
Atrial fibrillation
Supraventricular tachycardia
Premature AV junctional complexes
AV junctional rhythm
Premature ventricular complexes
Ventricular tachycardia
Ventricular fibrillation
First-degree AV block
Second-degree AV block
Third-degree AV block or complete heart block
The clinical treatment of dysrhythmias depends on the type of rhythm disturbance. Drugs used to treat dysrhythmias reduce automaticity of ectopic foci, alter conduction velocity or cardiac membrane responsiveness, inhibit the effects of circulating catecholamines, prolong action potential, or inhibit the influx of calcium ions [63].
Dysrhythmias may result from internal or external forces. Common internal forces include hypoxia, electrolyte imbalances, anemia, acidosis, diseases of the myocardium, and atherosclerotic processes [2,60]. Contributing external forces are stress, exercise, pain, hypovolemia, and caffeine ingestion.
Cardiac muscle cells have the capability of automaticity (i.e., the ability to fire without stimulation), allowing the cell to serve as a pacemaker or to cause premature beats. Each heartbeat creates distinctive wave forms: P, QRS, and T. Each QRS should correspond to the pulse. An action potential is the electrical activity that occurs in a cell by the movement of ions across cell membranes. During the resting state, the cell is polarized, with a balance of positive and negative ions on either side of the membrane, maintained by the sodium-potassium pump [60,61].
Depolarization (contraction) requires a change from a negative to positive state. During this stage, sodium ions enter rapidly, opening the sodium channels; calcium is allowed in, but potassium is not allowed to cross. A threshold potential is reached when the cell becomes less negatively charged, creating an action potential and depolarizing cardiac muscle cells. Calcium enters the cell and causes contraction of the cardiac muscle [60].
Repolarization then occurs. Sodium channels close, the cell begins to gain a negative charge, and the sodium-potassium pump restores the ions to proper concentration. The sequence is repeated for each heartbeat [60].
The refractory period is a certain stage in the conduction cycle in which there is resistance to stimulation. The absolute refractory period occurs from the beginning of Q to the middle of the T wave; no stimulus of any size can cause another impulse to occur during this period. The relative refractory period begins at the middle of T and continues to the end of the T wave; a larger than usual stimulus can stimulate the muscle cells to contract during this stage. For example, a PVC may occur at the same time as a T wave, resulting in biventricular tachycardia or ventricular fibrillation [60].
There are two major categories of dysrhythmias based on the underlying etiology: those caused by an alteration in impulse formation and those caused by an alteration in conductivity [76,77]. Dysrhythmias caused by an alteration in impulse formation may display tachycardia or bradycardia and the rhythm may be regular or irregular. There are ectopic beats (extra impulses), including premature atrial contractions, premature junction contractions, junction escape beats, and PVCs. On the other hand, alterations in conductivity result in heart blocks. This is the blockage of an impulse through one of the bundle branches, causing the impulse to retrograde, re-entering the other bundle branch and causing a premature beat. Cardiac dysrhythmias are further classified according to the site of impulse formation or the site and degree of conduction block [76,77].
Sinus Rhythms
In normal sinus rhythm, impulse originates at the SA node and travels the normal pathway with no delays. The waveforms are uniform and are of a fixed duration. The P wave represents atrial depolarization and the waveform is normal, smooth, and upright.
A sinus rhythm that varies in rate during inspiration (faster) and during expiration (slower) is common in the very young and the very old. This is referred to as sinus arrhythmia.
Sinus tachycardia has the same configuration as normal sinus rhythm, but the rate is greater than 100 bpm. This can be an early warning sign of cardiac dysfunction, such as heart failure. Patients with sinus tachycardia may be asymptomatic or may experience a "racing" feeling, syncope, and/or dyspnea. This dysrhythmia may be related to episodes of hypotension [76,77].
Sinus bradycardia also has the same configuration as normal sinus rhythm, but the rate is less than 60 bpm. Some patients do not experience any associated symptoms, but others may display a decreased level of consciousness, syncope, and hypotension [76,77].
When all configurations are normal but there is a drop in one or more complete complexes, this is referred to as sinus arrest or sinus block. With sinus block, the measurement of rate and rhythm will be exact, even with missing complexes. Sinus arrest is associated in a non-exact measurement of rate and rhythm with the missing complex. Symptoms may not be present unless the pause is great enough to decrease cardiac output; if cardiac output is impaired, the patient will experience the same signs and symptoms as bradycardia.
Sinus node dysfunction occurs when the SA node is unable to pace the heart normally. Referred to as sick sinus syndrome, sinus node dysfunction encompasses a group of disorders that cause problems with formation, transmission, or conduction of the impulse and are often found in older patients. ECG characteristics of sick sinus syndrome include changes in the type of rhythm, the pacemaker site, and/or the rate. The patient may experience some or all of the following:
Sinus bradycardia or sinus arrhythmia
Sinus pauses or sinus arrest
Atrial tachycardia (e.g., atrial fibrillation, atrial flutter, atrial tachycardia)
Brady-tachy syndrome (i.e., alternating periods of bradycardia and tachycardia)
Signs and symptoms of sick sinus syndrome include dizziness, light-headedness, syncope, fatigue, and other signs of decreased cardiac output [76,77].
Atrial Dysrhythmias
With atrial dysrhythmias, the impulse originates in the atrial tissue (ectopic) outside the normal conduction system (SA node). For example, premature atrial contractions are characterized by an ectopic beat that comes early in the cycle with the same configuration as normal beats. This condition is usually asymptomatic and benign [76,77].
Atrial tachycardia or paroxysmal supraventricular tachycardia is a fast rate with unidentifiable, normal P wave or P wave with a different configuration. This condition has a sudden onset and sudden termination, usually related to the re-entry mechanism around the AV node. It is seen more often in women than men. Signs and symptoms include palpitations, "racing" heart, anxiety, dizziness, dyspnea, angina, diaphoresis, extreme fatigue, and polyuria.
Atrial flutter usually has a regular rhythm (though it may be irregular) with a saw-toothed wave on ECG instead of normal P waves. It is believed to be the result of an intra-atrial re-entry mechanism. Some patients will not experience any symptoms, but others may report palpitations or "fluttering" in the chest. If rapid ventricular response is present, patients will display signs of decreased cardiac output due to the loss of "atrial kick" and decreased ventricular filling time.
Chaotic atrial activity causing the atria to quiver (400 to 600 impulses) instead of contracting normally is referred to as atrial fibrillation. It may be intermittent or a chronic rhythm disturbance. In patients with atrial fibrillation, rapid impulse firing from the atrial wall bombards the AV node, resulting in a wavy baseline between rate and rhythm, no visible consistent P waves, and an irregular ventricular response pattern. Atrial fibrillation is most likely to increase the ventricular rate, which decreases diastolic filling time and cardiac output. Clinical signs and symptoms depend on the ventricular response. Peripheral pulses will be irregular and variable in quality. Signs and symptoms of decreased cardiac output are common, including hypotension, shortness of breath, fatigue, angina, syncope, and heart failure. Patients with atrial fibrillation are at high risk for thromboembolic formations due to pooling of blood in the atria and the absence of "atrial kick." Synchronized electrical cardioversion is often necessary to correct atrial fibrillation [76,77].
Junctional Dysrhythmias
Premature junctional contraction is an early impulse in the cycle, originating in the AV node. This dysrhythmia originates in the AV node with inverted P waves that may fall before, during, or after the QRS. It is a failsafe mechanism when the SA node does not fire, but it should not remain as the dominant pacemaker. Depolarization of the atria is through retrograde conduction, so any visible P wave will be inverted on the ECG strip. The P wave may be absent or inverted before or behind the QRS. Premature junctional contraction is usually asymptomatic and presents with a slower heart rate (40 to 60 bpm). Some patients will experience signs and symptoms of decreased cardiac output from the absence of "atrial kick" and decreased myocardial tissue perfusion leading to ischemia and potentially signs of heart failure [76,77].
Accelerated junctional rhythm and junctional tachycardia are distinguished by different rates. Accelerated junctional rhythm has a faster than normal (60 to 100 bpm) rate of the AV node, and junctional tachycardia displays an even faster rate (greater than 100 bpm).
Ventricular Dysrhythmias
As may be assumed, ventricular dysrhythmias originate in the ventricles (i.e., idioventricular rhythm). They are denoted by wide and bizarre QRS complexes that are greater than 0.12 seconds in duration with increased amplitude, abnormal ST segment, and a T wave that has an opposite deflection from the QRS complex. The P wave will have no relationship to the QRS complex. The inherent rate is 20 to 40 bpm [76,77].
The most common dysrhythmias are PVCs. These dysrhythmias come early in the cycle and are frequently caused by hypokalemia [76,77]. PVCs are usually clinically insignificant in older adults, but frequent, recurrent, or multimodal PVCs indicate myocardial irritability and may precipitate lethal dysrhythmias. If present, signs and symptoms include a feeling of "skipped beats," chest discomfort, dyspnea, hypotension, and dizziness. The incidence is greatest following myocardial ischemia, MI, hypertrophy, or infection. These dysrhythmias may be unifocal or multimodal (i.e., coming from different sites in the ventricular wall). PVCs may also follow specific patterns [76,77]:
Bigeminy: Every other beat is a PVC
Trigeminy: Every third beat is a PVC
Couplets: Two PVC beats together
Triplets: Three PVC beats together
Salvo: Three to six PVC beats in a row
Ventricular tachycardia is a rapid ventricular rhythm disturbance defined as three or more consecutive PVCs. It may be a short burst or sustained. The rhythm is usually regular with a rate greater than 100 bpm, and the typical cause is re-entry mechanism. Signs and symptoms include fluttering in the chest, palpitations, shortness of breath, signs of decreased cardiac output, hypotension, loss of consciousness, and no palpable pulses. If left untreated, ventricular tachycardia may deteriorate into lethal rhythm [76,77].
Ventricular fibrillation is a rapid, chaotic ventricular rhythm causing the ventricles to quiver. The ECG of a patient with this dysrhythmia shows chaotic, irregular, bizarre complexes with no discernible rate or rhythm. In ventricular fibrillation, the heart does not pump and cardiac arrest occurs. Without effective treatment (i.e., defibrillation and cardiopulmonary resuscitation [CPR]), death occurs.
Idioventricular rhythm is characterized by the absence of normal complexes. P waves, if present, are not associated with the QRS, which appears wide and bizarre (as with PVCs). The overall rate is slow (20 to 40 bpm). The patient with idioventricular rhythm typically loses consciousness, and other signs of decreased cardiac output may be present. It is a potentially lethal arrhythmia that may follow defibrillation, ventricular fibrillation, or ventricular tachycardia [76,77].
Atrioventricular Conduction Blocks (Heart Blocks)
Atrioventricular conduction blocks are conduction defects that delay or block transmission of the sinus impulse through the AV node. These arrhythmias may be the result of an injured or diseased SA or AV node area or from increased vagal tone [76,77]. Heart blocks range from benign to severe, but even the more benign conditions should be monitored for progression to harmful levels.
First-degree AV block is a benign condition and is not considered a true block. It is characterized by a prolonged but constant PR interval (greater than 0.20 seconds in duration) and an otherwise normal sinus rhythm. Though it is usually asymptomatic, it can progress to a higher level block.
Type 1 second-degree AV block (also known as Mobitz I or Wenckebach) presents as a cyclic pattern of complexes with a progressively prolonged PR interval. Eventually, one QRS is totally blocked or dropped [76,77]. At first, the patient will be asymptomatic. However, when the rate drops, signs and symptoms of decreased cardiac output are observed.
Type 2 second-degree AV block (also referred to as Mobitz II or classical) is usually seen in patients with CHD and anterior wall MI. This type of block displays as more than one P wave to every QRS complex and a constant PR interval. The signs and symptoms depend on the ventricular rate.
Third-degree AV block (also called complete heart block) occurs when P waves and QRS complexes (ventricular rhythm) are regular but are in no way related to each other. There may or may not be more P waves than QRS complexes, and the PR interval constantly varies. Signs and symptoms are associated with bradycardia, as the rate can be as low as 30 bpm, and decreased cardiac output, including light-headedness, confusion, and syncope. This type of block requires intervention and can be life-threatening, with an 80% mortality rate [76,77].
In right and left bundle branch blocks, the electrical impulse is conducted slowly through the ventricles, causing a widened QRS complex (greater than 0.12 seconds) and a rabbit ear configuration on QRS. This is usually a benign, asymptomatic process unless it is coupled with an AV block.
A variety of other miscellaneous atrioventricular conduction blocks may occur, with various origination sites (e.g., SA node, atrial cell, AV node) and ECG presentations (e.g., normal Ps, no Ps, inverted Ps). These blocks may be benign unless the ventricular rate is altered, in which case the patient would experience symptoms of bradycardia or tachycardia [76,77].
Nursing assessment of the patient with known or suspected cardiac arrhythmia should include evaluation of vital signs and assessment for symptoms of decreased cardiac output. The ECG should be analyzed to monitor the pattern for rate, rhythm, P wave morphology, calculation of intervals, assessment of the ST segment, and determination of rhythm [17].
Diagnostic tests used most commonly to identify and categorize cardiac arrhythmias include single-lead telemetry (usually lead II) and electrophysiologic studies. There are no specific laboratory tests available to diagnose cardiac dysrhythmias, but they may be helpful in determining the underlying cause (e.g., hypoxia, electrolyte imbalances, acid-base imbalances, serum drug levels).
Antidysrhythmic medications may be prescribed to help control cardiac rhythm abnormalities [78]. Collaborative care between the nurses and physician is vital, and standing orders are needed for the treatment of lethal arrhythmias. These patients should be regularly monitored, and oxygen may be administered as necessary. In cases of chronic arrhythmias, placement of a permanent pacemaker is usually indicated. In emergency situations, countershocks are often necessary.
Permanent Pacemakers
Many dysrhythmias are treated with the placement of a permanent pacemaker. The most common types of pacemakers in use are atrial demand pacemakers, ventricular demand pacemakers, dual-chamber pacemakers, and ventricular-paced/dual-sensing single chamber pacemakers. Demand pacemakers are used most often. These devices are set at a rate that is individualized for each patient and begin to fire when the patient's heart rate falls to or below a set rate [37].
In atrial demand pacemakers, the spike occurs before each P wave, while the spike occurs before each QRS complex with ventricular demand pacemakers. AV sequential pacemakers initiate two spikes: one before the P wave and one before the QRS [37].
Although pacemakers are generally safe, problems may be encountered. The most common are [37]:
Failure to sense: The pacemaker does not sense the patient's rhythm and continues to send an impulse. When this dysfunction occurs, the ECG will show a spike at regular intervals throughout the patient's regular rhythm.
Failure to pace: The pacemaker fails to begin firing when the heart rate drops below the set rate. The ECG will show whatever rhythm occurred that caused the need for the pacer without the associated spike.
Failure to capture: The pacemaker fires in a regular pattern but the ventricle does not depolarize. The ECG will show a spike without any QRS.
Countershock
Countershock is used to detect and interrupt cardiac rhythms that compromise cardiac output. This approach causes total depolarization of all cardiac cells, wiping out the dysrhythmia and allowing the SA node to resume its normal rhythm by delivering an electrical stimulus to the heart. The two types of countershock are rapid defibrillation and synchronized cardioversion [79]. Defibrillation is the emergency treatment for ventricular fibrillation and consists of electrode pads or paddles attached to cables that allow for an electrical shock to be externally administered to the patient's heart. Effective defibrillation is partially dependent on the presence of adequate high-energy phosphate stores in the myocardium. If phosphate stores are adequate, the heart will have the necessary energy resources to restart in an effective rhythm. Rapid arrhythmias, such as ventricular fibrillation, consume high-energy stores very quickly; for that reason, it is critical to shock the heart out of ventricular fibrillation before the energy sources are depleted.
In synchronized cardioversion, the machine delivers the shock in synchrony with the patient's own rhythm; this avoids the risk that the shock will fall during the vulnerable period of repolarization (i.e., during the T wave) and send the heart into ventricular fibrillation. Cardioversion has been found to be effective for arrhythmias caused by re-entry mechanisms (e.g., those underlying most episodes of monomorphic ventricular tachycardia). Setting up for cardioversion usually takes a few minutes longer than preparing for defibrillation. The machine uses a sophisticated sensor to identify the highest point of the R wave in the patient's cardiac cycle to time the delivery of the synchronized shock. For the machine to properly identify the R wave, electrodes (not the "quick-look" paddles) must be used. Additionally, delivery of a direct current shock through the chest wall is extremely painful; if possible, the patient should be sedated before the shock is administered. If the patient's status is rapidly deteriorating or is so unstable that the delay of a few minutes to set up and prepare the patient for synchronized cardioversion jeopardizes his/her status, defibrillation should be used instead.
Inflammatory diseases of the heart are defined as conditions causing inflammation of any layer of cardiac tissue (i.e., the endocardium, myocardium, or pericardium); these diseases can damage the valves, muscle, or pericardial lining. Conditions include rheumatic heart disease, endocarditis, myocarditis, and pericarditis [2,80].
Rheumatic heart disease is a chronic condition characterized by a slowly progressive valvular deformity that follows an acute or repeated episode of rheumatic fever (i.e., inflammatory disease from a beta-hemolytic streptococcus). In the acute stage, valves become red, swollen, and inflamed, with lesions developing on the leaflets. In the chronic stage, scar tissue develops, leaflets become rigid and deformed, and stenosis or regurgitation may develop. Left heart valves are affected more often, most commonly the mitral valve. Signs and symptoms include [2,80]:
Precordial chest discomfort
Tachycardia
Evidence of heart failure
Pericardial friction rub
S3 (ventricular gallop) or S4 (atrial gallop)
Murmur from mitral or aortic regurgitation
Cardiomegaly
Pericardial effusion
Nursing Assessment
Patients with rheumatic fever should be monitored via vital signs, lung sounds, heart sounds, apical pulse, and symptom analysis of any chest discomfort. Diagnostic testing may include CBC, C-reactive protein (as an indicator of inflammation), erythrocyte sedimentation rate (ESR), and antistreptolysin titer [17,18,19].
Nursing Management
Rheumatic fever can be prevented by the early identification and treatment of group A streptococcal tonsillopharyngitis [81]. If rheumatic fever does develop, anti-inflammatory agents (e.g., salicylic acid, corticosteroids) are used to control the acute symptoms while the underlying infection is also treated. If heart failure results from rheumatic heart disease, the usual treatment is diuretics and bed rest [81].
Endocarditis may result from invasion of pathogenic organisms or injury to the lining of the heart. Infective endocarditis is usually caused by streptococci or staphylococci. Any part of the endothelial lining of the heart may be affected, but the mitral valve is most susceptible. The condition may be classified as either acute or subacute. The process begins with platelet-fibrin vegetation on healthy valves (acute) and on damaged valves or endocardium tissue (subacute). Signs and symptoms of infective endocarditis are murmur, cough, shortness of breath, anorexia, abdominal pain, anemia, splenomegaly, fever (greater than 101.5o F), chills, night sweats, flu-like symptoms, joint pain, mitral valve murmur, arterial emboli, and petechiae in the skin or mucous membranes. With arterial emboli, the patient may present with decreased or absent pulses in the affected extremity, cerebrovascular symptoms related to stroke, abdominal pain related to bowel or spleen infarctions, back pain related to infarction of the kidney, or chest pain related to MI [60,61].
Nursing Assessment
All patients should have their vital signs, symptoms, and complaints assessed. In patients with known or suspected endocarditis, nutritional status, skin status, heart sounds, peripheral pulse, and capillary refill should be evaluated. The spleen should be palpated. Diagnostic testing includes CBC, immune testing, blood culture, urine testing (to identify increased creatinine, hematuria, and protein), and ECG.
Nursing Management
Medical management is critical because infective endocarditis is usually fatal if untreated. The primary goal is to treat the infective organism, if known. When the organism is not known, nafcillin, ampicillin, and gentamicin are often used in empiric combination therapy. Antipyretics and/or anti-inflammatory agents may be given to control symptoms. Treatment is continued for at least four weeks and may be extended eight weeks or longer to achieve a cure [66].
Defined as an inflammatory disorder of the heart muscle unrelated to CHD or MI, myocarditis may be caused by viruses, bacteria, protozoa, radiation, chemicals, pharmacologic agents, or metabolic disorders. Immunologic response may be due to the effects of radiation, chemical poisons, drugs, burns, or most commonly in the United States, coxsackie B enterovirus. The inflammatory process causes local or diffuse swelling and damage to the cells of the myocardium, abscesses in interstitial tissues from infectious agents, and pinpoint hemorrhage in layers of the heart. If the patient's condition deteriorates, dilated cardiomyopathy may result, with accompanying weakening of the heart muscle and decreased contractility. Several months or years after the initial myocarditis, the individual may develop clinical manifestations of CHF with ventricular enlargement and dysfunction. Progressive deterioration is usually followed by death within four years.
Signs and symptoms depend on the degree of cardiac damage [60,61]. At first, patients may be asymptomatic. Signs of inflammation include fever, chills, fatigue, malaise, dyspnea, palpitations, and arthralgia. If the patient progresses to heart failure, he or she may experience tachycardia, dysrhythmias, S3 or S4 gallop, systolic murmur, transient pericardial friction rub, cardiomegaly, and ECG abnormalities.
Nursing Assessment
Aside from monitoring vital signs, patients with myocarditis require analysis of complaints and pain, evaluation of the ECG pattern, and assessment of heart sounds. Diagnostic tests include ESR, CBC, antiviral antibodies, ECG, heart catheterization with biopsy of heart muscle, and serum immunoglobulin cultures.
Nursing Management
Myocarditis and related symptoms are treated with antipyretics, antidysrhythmics, anticoagulants, and corticosteroids or immunosuppressive drugs [66,67]. Digoxin may be administered if heart failure occurs.
Pericarditis is defined as an inflammation of the pericardium. When inflammatory mediators are released, vasodilation occurs, causing hyperemia and edema. Increased capillary permeability allows plasma proteins and fibrinogen to gather in the pericardial space [60]. Exudate is formed that may contain red blood cells or be purulent if infective. The inflammation, whether acute or chronic, is frequently not properly diagnosed and may result in delayed treatment and even death. Pericarditis may be caused by trauma, malignancy, anticoagulants, bleeding disorders, systemic diseases, MI, or illicit drugs, as well as infection with viruses, bacteria, fungi, or parasites. Common infectious causes include staphylococci, streptococci, gram-negative bacilli (e.g., Neisseria, Salmonella), and certain fungi (e.g., Histoplasma, Coccidioides).
Pericarditis may be primary or secondary to other disease processes. It is often viral in origin and affects more men than women. It is also a frequent complication of end-stage renal failure and may be seen after MI or following open-heart surgery.
Fibrosis and scarring may restrict the heart's ability to function effectively, and pericardial effusions may develop. Chest pain occurs due to inflammation of nerve fibers. Pericarditis may cause the life-threatening emergency of cardiac tamponade, an accumulation of a large amount of fluid in the pericardial sac that prevents the heart from filling and consequently reduces cardiac output dramatically. Cardiac tamponade usually requires pericardiocentesis, an immediate surgical procedure to empty the pericardial sac. Constrictive pericarditis may develop over time and requires surgical removal of the pericardium. Acute pericarditis lasts two to six weeks and is characterized by [60,61]:
Sharp chest pain (rapid onset) that worsens with deep breath, cough, or change in position (particularly lying supine)
Dyspnea
Dry cough
Fever (low grade)
Chills and perspiration
Pericardial friction rub, usually on expiration
ECG abnormalities
Pulsus paradoxus
Jugular venous distention
Cardiac tamponade or pericardial effusions
Nursing Assessment
Patients with pericarditis should have their pain analyzed to determine what worsens or relieves it (if anything). In addition, vital signs, lung sounds, and respiratory status should be regularly monitored [18,19]. Diagnostic testing includes ESR, ECG, echocardiogram, hemodynamic monitoring, chest x-ray, computed tomography, and MRI [17].
Nursing Management
Treatment includes rest and close observation for the development of cardiac tamponade and treatment of the underlying infection. Nonsteroidal anti-inflammatory agents are used to control pain. Steroids for pain are used when other agents are not effective. When necessary, surgery is performed [61].
Valvular heart disease encompasses any conditions that interfere with the unidirectional blood flow within the heart. The underlying cause may be either acute (e.g., endocarditis, calcium deposits) or chronic (e.g., rheumatic heart disease). Valvular heart disease presents as stenosis, regurgitation, incompetency of the valve, or a combination of these factors. A stenotic valve impedes blood flow forward, while an incompetent valve does not close after blood has entered the chamber and should be moving forward. Both stenotic and incompetent valves may lead to heart failure. Specific valvular disorders include [2,80]:
Tricuspid stenosis
Tricuspid insufficiency
Pulmonic stenosis
Pulmonic insufficiency
Mitral stenosis
Mitral insufficiency
Mitral valve prolapse
Aortic stenosis
Aortic insufficiency
Treatment is centered on teaching the patient about the need for prophylactic antibiotics while the condition is being corrected. Dentists and other physicians should be informed about the diagnosis before planning invasive procedures.
Valvular stenosis involves fusion of the valve leaflets, producing a narrow opening and rigidity. The fusion may result from scarring of the valves caused by endocarditis, MI, or calcification.
Blood flow is impeded, resulting in decreased cardiac output and impaired ventricular filling, ejection fraction, and stroke volume. The mitral, tricuspid, aortic, or pulmonary valves may be affected by stenosis [80].
Regurgitation is caused by insufficient or incompetent valves preventing complete closure and allowing backflow of blood. It may be the result of deformity or erosion of the valve by vegetative lesions of bacterial endocarditis, scarring or tearing from MI, and/or cardiac dilation [80].
Hemodynamic changes occur with valvular heart disease. Blood volume and pressure are reduced in front of the affected valve, while the chambers behind the affected valve hypertrophy due to the increased pressure of the blood flow. Cardiac output falls as compensatory mechanisms become ineffective and the heart begins to fail. Increased muscle size leads to increased oxygen demand. This, coupled with decreased blood supply to the enlarged muscle, leads to heart failure. Infarction and loss of functional muscle also occur. When the mitral valve collapses back into the atrium causing regurgitation of blood from the ventricle to the atrium, this is referred to as mitral valve prolapse [2,80].
Signs and symptoms of stenosis include pulmonary congestion, dyspnea, pulmonary hypertension, dizziness, fatigue, tachycardia, murmur or rub, an S3 or S4 heart sound, and angina. Pulmonary stenosis may cause venous distention and swelling in the lower extremities [2,80].
The signs and symptoms of regurgitation may vary based on the affected valve. Mitral valve regurgitation may cause dyspnea, pulmonary hypertension, decreased cardiac output, dizziness, fatigue, tachycardia, angina, murmur or rub, and/or an S3 or S4 heart sound [2,80]. If the aortic valve is involved, the patient may present with wide pulse pressure, hyperkinetic (strong, bounding) peripheral pulses, signs and symptoms of CHF, and angina.
Assessment of the patient with valvular heart disease includes vital signs, heart sounds, lung sounds, symptom analysis of complaints and pain, respiratory status, exercise tolerance, presence of edema, and peripheral pulses. ECG, echocardiogram, chest x-ray, and cardiac catheterization may be necessary in order to make a definitive diagnosis [17].
Medications for the management of valvular disease include digitalis, diuretics, beta blockers, class I or III antidysrhythmics, and prophylactic antibiotics prior to invasive procedures. Abruptly stopping beta blockers may cause symptoms to return. Close observation for progression of the disease and angina is necessary. Observe for signs and symptoms of CHF and treat as necessary. Pre- and postoperative teaching should begin when medical treatment is no longer effective and surgical intervention necessary [63,67].
Hypertrophic cardiomyopathy, also known as idiopathic hypertrophic subaortic stenosis, is characterized by an enlarged heart but small ventricular chambers that are resistant to filling during diastole. The atria compensate for this by expanding. The condition is frequently observed in young adulthood, and sudden death is common. Death frequently occurs during strenuous exercise and is due to dysrhythmias. Signs and symptoms include dyspnea, inadequate cardiac output, angina, fatigue, syncope, abnormal ECG tracings, dysrhythmias, and abnormal chest x-ray showing left atrial enlargement or general cardiac enlargement [41].
Hypertrophic cardiomyopathy presents a challenge to manage because many of the available therapies for heart problems are contraindicated in its treatment. Treatment includes avoidance of strenuous exercise, tachycardia, and hypotension, as these events increase the likelihood of sudden death. Beta blockers or calcium channel blockers are usually prescribed, and placement of a pacemaker may be indicated [62]. Heparinization and cardioversion in the presence of atrial fibrillation is done. Other treatments include digitalis and diuretics for patients who develop CHF and do not have the obstructive component of the disease. Antibiotic prophylaxis is prescribed for any invasive procedures, including diagnostic screenings, to prevent the development of infective endocarditis of the aortic valve, mitral valve, or septum. Surgery may be performed to replace the mitral valve or to remove a wedge of the hypertrophied myocardium from the left ventricular septum [41,62].
Nurses should provide patient teaching and counseling regarding the diagnostic process, illness prevention, and treatment options. It may also be necessary to prepare patients for the lifestyle changes that will be required.
Shock occurs when tissue anoxia develops from insufficient blood flow to vital organs. It may be the result of decreased cardiac output and/or impaired blood flow. Shock is described according to its degree as preshock, reversible shock, or irreversible shock. Preshock implies that the patient is in a stage that will progress to shock if not treated. Reversible shock is treatable, but in irreversible shock, all available treatments fail and the patient has no hope for recovery. In cardiogenic shock, severe heart failure is the cause, usually due to acute MI [40].
Shock is a syndrome or group of symptoms and signs. Any two of the following signs must be present in order to make a clinical diagnosis [40]:
Decreased urinary output of less than 30 mL/hour with less than 30 mEq of sodium per liter of urine
Signs of poor tissue perfusion (e.g., pale, cool skin)
Systolic blood pressure less than 90 mm Hg for at least 30 minutes
Metabolic acidosis
Impaired state of consciousness (e.g., agitation, somnolence, confusion, coma)
PCWP greater than 15 mm Hg
By assessing the patient and reporting findings, the nurse plays an important role in aiding early diagnosis and treatment. By assessing early signs of heart failure, the nurse can help prevent progression to a severe state of shock, which is associated with a high mortality rate [39].
Cardiogenic shock may be caused by any condition that causes the heart to fail. As noted, the most common cause of cardiogenic shock is acute MI. Other problems with the myocardium, such as myocarditis or cardiomyopathy from various causes, and dysrhythmias that produce bradycardia or tachycardia may also lead to cardiogenic shock. Mechanical factors that impair blood flow through the heart, such as valvular problems, rupture of the interventricular septum, ventricular aneurysm, papillary muscle rupture, and trauma, can lead to cardiogenic shock [39].
The clinical manifestations of heart failure are important for the nurse to assess so that medical interventions and nursing interventions can be instituted in the preshock state. Clinical manifestations typically observed in cardiogenic shock are produced by sympathetic activity or occur over a period of time. The patient may have mental symptoms such as restlessness, confusion, or stupor. Body temperature is decreased, and the skin will become moist and cool. The pulse is rapid and weak, and blood pressure, pulse pressure, and urinary output will decrease [39,40].
The goals of treatment are determining and eliminating the cause, determining and treating the condition that precipitated the heart failure, and improving the ability of the heart to pump blood [37,39,40]. The medical plan of therapy is based on the cause of the cardiogenic shock. If the cause is mechanical, such as ventricular septal defect, papillary muscle dysfunction, or ventricular aneurysm, immediate surgery may be indicated. In some cases, an attempt will be made to stabilize the patient using an intra-aortic balloon pump to provide assistance to the heart. The balloon is placed in the descending aorta and inflated during diastole to help push the blood forward to the peripheral circulation and back to fill the coronary arteries. The balloon is deflated during systole, which reduces the resistance against which the heart has to pump.
The major responsibilities of the nurse are assessing changes in the patient's condition, providing comfort, and promoting a sense of security. Nursing measures for the patient with heart failure are also appropriate for the care of the patient with cardiogenic shock [17].
Cardiac arrest indicates that the heart has stopped beating. The term is used more loosely, however, for the practical purposes of determining the necessity for CPR. Deepening cyanosis of rapid onset, absence of heart sounds, and a lack of detectable pulses in the major vessels are sufficient findings to diagnose cardiac arrest. Most cardiac arrests occur before the victim reaches the hospital, and because so many deaths occur before hospitalization, the American Heart Association and the American Red Cross have developed community education programs and training in CPR for the public as well as healthcare professionals.
The initial approach to cardiac arrest depends on whether the patient is monitored. Hospitals and other health agencies have trained teams of personnel and guidelines to follow in the event of a cardiac arrest. All personnel are trained in basic cardiac life support, and special teams are trained in advanced cardiac life support. When cardiac arrest occurs, basic cardiac life support is initiated and the emergency plan developed by the agency is activated.
If the patient is monitored and the cause of the arrest is ventricular fibrillation, electrical countershock is performed. If countershock is unsuccessful, CPR is initiated and the procedure for unmonitored ventricular fibrillation is followed. If these procedures are successful, vasopressor and antidysrhythmic therapy is initiated [39,79].
If ventricular fibrillation is diagnosed and confirmed by ECG after CPR has been initiated, countershock is used as soon as the equipment is available. If countershock is unsuccessful, an endotracheal tube is usually inserted and oxygen therapy initiated. An IV line is established, epinephrine is administered intravenously or though the endotracheal tube, and defibrillation by countershock is attempted again. Sodium bicarbonate may be administered, depending on the length of time after cardiac arrest and the probability of acidosis. Defibrillation by countershock is attempted again.
If the cause of the arrest is ventricular tachycardia, synchronized cardioversion (application for an electrical current at the time of the QRS complex in the cardiac cycle) is performed. Less current is used than for countershock by defibrillation [79].
If cardiac arrest resulted from ventricular asystole, or ventricular asystole resulted from other causes of cardiac arrest, CPR is initiated or continued, an endotracheal tube is inserted, oxygen therapy is initiated, and an IV infusion is started. Drug therapy is started to initiate a rhythm. Drugs may include epinephrine, sodium bicarbonate, atropine, calcium chloride, or isoproterenol. A temporary pacemaker may be inserted to attempt to restore cardiac rhythm. The prognosis for successful resuscitation of cardiac arrest from asystole is poor [39,66].
During the early postoperative period following cardiac surgery, adequate oxygenation is essential, because hypoxia can stimulate dangerous dysrhythmias. The major cardiac surgical procedures include coronary artery bypass graft, angioplasty, valve replacement, heart transplantation, implantation of devices, and repair of dissections and aneurysms.
In coronary artery bypass surgery, a graft consisting of the saphenous vein, internal mammary artery, or both brings a new blood supply to the distal position of the stenotic coronary artery and to the ischemic myocardium. The surgeon connects the saphenous vein graft to the aorta proximally and to the coronary artery distally. Blood flowing into the aorta then passes through the new graft to the coronary artery, restoring the blood supply.
During the preoperative period, prepare the patient physically and psychologically for the surgery, including teaching regarding the tubes that will be present after surgery. Patients should be prepared for the surgical procedure in terms of anatomical explanations, procedures, and deep breathing and coughing exercises during the postoperative period.
The goal of care in the immediate postoperative period is the prevention or early detection of complications of cardiac surgery. Assessment of arterial pressure, pulmonary artery pressures, heart rate, and heart rhythm is done every 15 minutes. A slow rate can indicate heart block. Chest drainage and urinary flow are measured hourly. Blood counts, clotting studies, and ABG measurements are performed regularly. Assessment of neurovascular, abdominal, and pulmonary functions and the circulatory function of the operated leg are performed as ordered. The patient's fluid and electrolyte balances should be monitored. Pulmonary status is particularly important, as atelectasis in the early postoperative period is documented by decreased breath sounds and fever. When patients are extubated, they should perform deep-breathing and coughing exercises every two hours and use an incentive spirometer hourly. Patients on bed rest should perform range-of-motion exercises. Activity will be gradually increased to full accomplishment of activities of daily living.
Offer appropriate nutrition during the postoperative recovery period (i.e., clear liquids until the nausea from anesthesia subsides, then high-protein foods in frequent small feedings for the first few days). Custards, puddings, and milkshakes provide calories as well as protein.
Discharge teaching begins the fifth postoperative day and includes a clear description of what normal recovery includes, which signs and symptoms to report, and the dosages and side effects of medications the patient will take home. Activity depends on the patient's response to exercise. Walking is encouraged, but patients should avoid lifting heavy objects for six weeks and driving a car for three weeks. Diet instructions depend on special needs, but all patients should follow a diet of no added salt for the first month after surgery, because the body is undergoing changes in its fluid balance. Adequate intake of nutrients is essential for proper wound healing, so patients should consume a diet high in protein, vitamins, and minerals. Eventually, a cholesterol-lowering diet plan can be instituted. Because the surgical leg tends to be edematous, instruct the patient to wear anti-embolism support stockings during the day and to remove them at night. The operated leg should be elevated when sitting until the swelling has subsided.
PTCA is an invasive technique used in the treatment of CHD. A balloon catheter is introduced into a vessel, and the balloon is positioned at the site of the atherosclerotic plaque. The balloon is inflated, compressing the plaque and re-establishing blood flow to the ischemic myocardium. The risk for MI is always present during this procedure.
Preoperative Care
Pre-angioplasty diagnostic tests include coronary angiogram, stress test, chest x-ray, ECG, blood chemistries, CBC, and glucose levels. Each patient is blood typed and screened in preparation for possible heart surgery. Medications are started, including anticoagulants and antiplatelet agents. A complete heart surgery preparation is done in the event an emergency coronary artery bypass graft must be attempted. The patient should not consume any food after midnight. The nursing assessment is an important activity in the preoperative period. Education for this procedure is just as important as for heart surgery. Explain the atherosclerotic process, the angioplasty procedure, and complications that can occur, particularly the potential need for bypass surgery if complications arise.
Postoperative Care
Four to six hours postprocedure, the most important aspect of nursing care is observing the patient for signs of coronary artery occlusion/ischemia, including chest pain, ECG changes, dysrhythmias, and hypotension. Monitor vital signs every 15 minutes for an hour and then every 30 minutes until stable. A 12-lead ECG should be taken every eight hours over the next 24 hours, and continuous ECG monitoring may be done. Assess the patient's peripheral circulation (e.g., color, warmth, pulses, sensation), mobility, and catheter insertion (for bleeding, hematoma, or infection) every hour for four hours, then every two hours for eight hours, and finally every four hours. Monitor the patient's urinary output every hour and breath sounds every two hours. Maintain pressure on the catheter insertion site until all bleeding has ceased, and keep the head of the bed below 30 degrees. Encourage fluids to assist in the elimination of contrast media used in the procedure. When discharged, teach the patient about the signs and symptoms to report, including chest pressure or heaviness, recurrence of angina pectoris, dizziness, or light-headedness.
Valve replacement involves the removal of the native valve and the insertion of a prosthesis. Three general categories of valves can be used: allografts, or human valves; xenografts, or animal valves; or artificial valves. Allografts are not in general use because they are difficult to obtain and offer no advantages over other valves. Xenografts originate from a pig or cow and are specially prepared for human use. An advantage of this type of valve is that there is little risk of clot formation on the valve leaflets. Artificial valves are prone to clot formation and require that the patient take anticoagulants for the rest of his or her life. An advantage of artificial valves is that they have greater longevity. Animal valves begin to deteriorate after 5 to 10 years and often require replacement.
Preoperative preparations for valve replacement are the same as those discussed for coronary artery bypass surgery. The nurse's teaching should include the need for anticoagulants following surgery and a special emphasis on coughing and deep-breathing exercises.
Postoperatively, the patient will require detailed assessment and monitoring, as described for the bypass patient. Adequate fluid and IV intake to maintain sufficient heart preload is important to ensure normal cardiac output. Prevent an overinfusion of fluids that may result in heart failure. Because atrial dysrhythmias are common, continuous ECG monitoring is indicated to provide early detection.
Heart transplantation is reserved for the patient with intractable cardiac disease when all forms of medical treatment have been exhausted. This includes extensive loss of ventricular function, severe CHD not amenable to bypass surgery, cardiomyopathy, severe valvular disease with cardiomyopathy, and congenital heart disease. Patient selection criteria typically include age younger than 55 years, emotional stability, good psychological support system, absence of insulin-dependent diabetes, absence of hyperlipidemia, normal pulmonary vascular resistance, and absence of other significant systemic disease.
It is important to note that the recipient's transplanted heart will have two P waves on the ECG, a higher resting heart rate than normal, and a slower heart response to exercise. Infection is the primary cause of death after heart transplantation, with the highest risk noted in the first three months after surgery. Rejection is the second most common cause of death and may be acute or chronic.
Cyclosporine is administered to the patient before surgery, and the other usual heart surgery preparations apply. Because the heart has been denervated and therefore does not respond to vagal or sympathetic stimulation, atropine cannot be administered if the patient has a bradydysrhythmia. The patient with myocardial ischemia also will not experience angina pectoris. Therefore, ischemic changes must be detected by ECG or myocardial enzyme studies.
Immunosuppressive drug therapy begins immediately after surgery. Monitor the patient for the side effects of immunosuppressive drugs and report them to the physician. These include abdominal discomfort, nausea, vomiting, headache, tremor, hypertension, and renal dysfunction. Long-term use of corticosteroids can cause osteoporosis, muscle weakness, sodium retention, glucose intolerance, increased infection risk, peptic ulcers, and mood swings. Protective isolation is maintained the first few weeks after surgery, during which period the patient is monitored for signs of rejection.
Discharge instructions are essential to successful rehabilitation. The patient must have knowledge of:
Purpose, dosage, and side effects of all medications and the need not to discontinue taking them without the physician's direction
Situations that increase the risk of infection and should therefore be avoided
Good care habits for teeth and skin
Early signs of infection
Treatment of injuries
The recommended diet (low in saturated fat and cholesterol)
Allowed activities
Follow-up appointments
The need to carry a medical information tag or card
An artificial heart is a device indicated for patients with intractable heart failure resistant to all known therapeutic measures. The major adjustment for the patient receiving an artificial heart is that he or she will always be connected to a drive unit that must be carried at all times.
Preoperative care for patients undergoing artificial heart implantation is similar to any major heart surgery. In the postoperative period, the patient should be monitored for mechanical failure. Hemodynamics and fluid management of the patient are different from that of other cardiac surgery patients. Hypertension should be avoided, with a target mean arterial pressure between 70 mm Hg and 90 mm Hg. If cardiac output falls, the drive pressure or heart rate is increased; inotropic drugs cannot be used. Creativity is necessary to assist the patient to manage mobility and maintain the integrity of the mobile unit. Rehabilitation is individually planned according to the patient's response to surgery.
Ventricular assist devices are mechanical pumps that can be used temporarily or over the long term to provide assistance to an overburdened, poorly functioning ventricle. Temporary assist is indicated for ventricular dysfunction in which recovery of the myocardium is anticipated, while long-term assist is indicated for permanent damage to the myocardium.
Many heart dysrhythmias can be treated successfully with pharmacologic therapy. However, medication is not helpful for treatment of chronic bradydysrhythmias; these patients will achieve lasting results with implantation of a permanent pacemaker. As discussed, these devices supply electrical impulses to the heart muscle to stimulate the heartbeat.
Patients with pacemakers should be monitored for mechanical failure and recurrence of the dysrhythmia. Patient teaching should include information about pacemaker location, appearance, function, and the signs and symptoms of failure (e.g., decreased heart rate, chest pain, dizziness, fainting, undue fatigue, shortness of breath). Patients should be advised to avoid excessive arm movements and reaching for five weeks postoperatively to prevent dislodging the pacing electrode. Check the wound every 30 minutes postoperatively for bleeding. Record fluid intake and output to ensure that the patient is well hydrated. Medication may be given for pain relief as needed. ECG monitoring may reveal device malfunction, including lack of capture, competition, and failure to pace. Additional patient teaching subjects include alarm sensitivity in airports, the need to take prophylactic antibiotics before any dental work, and the increased risk for bacterial endocarditis.
Historically, aortic aneurysms and dissections have had a high mortality rate, with little hope for patient survival. With current surgical techniques, however, more persons are surviving. There are two main approaches to the repair of aortic aneurysms: open repair and endovascular repair. Both aneurysms and dissections may be treated by resection of the affected section and replacement with a graft.
Because symptoms appear suddenly, the goals of nursing care in the preoperative period are to stabilize the patient's blood pressure and offer emotional support to the patient and family. The patient should be prepared for cardiac surgery.
Postoperatively, the patient should be constantly monitored for arterial pressure and maintenance of a pressure high enough for tissue perfusion but not so high that stress is placed on the suture lines. Nursing assessment includes evaluating circulation to the extremities, assessing for stroke, monitoring hourly urine output for decreased kidney perfusion, monitoring clotting studies to prevent coagulopathy, monitoring the ECG for signs of complete heart block, assessing breath and heart sounds, and monitoring pulmonary and right atrial pressures for signs of hypovolemia or heart failure. Analgesics should be administered liberally, especially if a thoracotomy incision was used. Emphasize breathing exercises as with any thoracic or cardiac surgery patient.
Patient A is White, 60 years of age, and works as a cab driver. While driving home after work, he develops an aching in his chest and slight, regular palpitations. The ache is still present when he goes to bed, when he wakes several times during the night, and when he gets up in the morning, seven hours after retiring. He drinks some soda water, but when the aching does not improve, he decides to go to the emergency department.
At the hospital, Patient A complains of chest pain accompanied by diaphoresis, slight shortness of breath, and nausea. Relief of pain is obtained with IV morphine sulfate. When the patient is admitted to the critical care unit (CCU), his symptoms are generally unremarkable except for recurrent pain.
Patient A experienced the usual childhood illnesses without rheumatic fever. As an adult, he has a history of hypertension (documented on discharge from the Army at 45 years of age and when hospitalized two years ago) that has not been treated. Past surgical history includes tonsillectomy and adenoidectomy as a child. A cataract was removed from his right eye two years ago.
Patient A's father died of an MI at 55 years of age. His mother is alive and well, although the patient does not know her age. Two brothers, 65 and 58 years of age, are alive and well. The patient lives alone and works approximately 72 hours per week. He has been married and divorced twice; the last divorce was four years ago. He has no children.
Upon admittance to the CCU, a full physical exam is conducted (Table 4). An ECG is done and shows ST elevation. Several laboratory tests are ordered, with the following results:
Serum cardiac enzymes:
CK: 164 IU/L
LDH: 219 IU/L
Serum glutamic-oxaloacetic transaminase (SGOT): 31 IU/L
CBC: Within normal limits
Electrolytes: Within normal limits
Urinalysis: Within normal limits
Based on the results of the assessment, Patient A is diagnosed with:
Acute anterolateral MI, generally uncomplicated
Atherosclerotic cardiovascular disease
Hypertension: Untreated for 15 years, probably essential hypertension given age at onset
PATIENT A'S PHYSICAL EXAM RESULTS
| Parameter | Findings | ||||
|---|---|---|---|---|---|
| General appearance |
| ||||
| Head and eyes |
| ||||
| Ears | Tympanic membranes intact | ||||
| Neck |
| ||||
| Chest | Clear to auscultation and percussion | ||||
| Abdomen |
| ||||
| Extremities | Peripheral pulses full, equal, and without bruits | ||||
| Genitourinary system | Within normal limits | ||||
| Neurologic status |
| ||||
| Cardiovascular system | Point of maximal impulse sixth intercostal space in the midclavicular line of normal intensity and duration, without heaves or thrills | ||||
| Vital Signs | |||||
| Blood pressure | 140/95 mm Hg | ||||
| Temperature | 98.6° F | ||||
| Heart rate | 55 bpm | ||||
| Respiratory rate | 18 breaths per minute | ||||
Patient A's vital signs are stable for the remainder of the day, with a sinus bradycardia of 56 bpm. Early in the morning the next day, the patient awakes with nausea and diaphoresis. His blood pressure has decreased to 90/60 mm Hg with sinus bradycardia of 40 bpm. PVCs are present. The patient is treated with 0.5 mg IV atropine sulfate twice, after which his heart rate increases to 70 bpm and his blood pressure increases to 130/68 mm Hg. Unifocal PVCs are then treated with 150 mg of amiodarone IV over 10 minutes followed by an amiodarone drip at 1 mg/minute for 6 hours, then 0.5 mg/minute for 12 hours.
Later in the day, Patient A's vital signs are:
Blood pressure: 130/90 mm Hg
Temperature: 98.4° F
Heart rate: 60 bpm
Respiratory rate: 18 breaths per minute
The patient has no further chest pain, but he reports that his nausea persists after meals.
Two days later, Patient A's LDH value rises to 310 IU/L; other enzyme levels remain essentially the same as the admission values. ECG shows ST elevation diminishing from previous levels. The amiodarone is discontinued without return of the PVCs. His vital signs remain stable, no further arrhythmias are noted, and his nausea is resolved. On day three, Patient A is moved out of the CCU and started on cardiac rehabilitation.
On day four, a treadmill test is done at 50% effort with negative results. Patient A is discharged on day seven. The medical plan is to continue treatment of his hypertension with propranolol. The patient plans to return to driving his cab, but for fewer hours per week.
List Patient A's major risk factors for CHD and discuss other possible risk factors for heart disease.
Discuss the pathophysiology of CHD and the signs and symptoms (i.e., classic physical exam findings) exhibited by the acutely ill patient during an MI. What are the common complications post-infarction?
What patient history points indicate the diagnosis of MI in Patient A's case?
Correlate the pathology, complications, and nursing care for a patient with MI with the patent's progress from the CCU to home.
Review the action, side effects, and specific nursing care for the drugs commonly used in the treatment of patients with MI, including:
Analgesics (e.g., morphine)
Sedatives (e.g., phenobarbital)
Antianxiety medications (e.g., diazepam)
Anticoagulants (e.g., heparin)
Laxatives/stool softeners
Vasopressors (e.g., norepinephrine)
Vasodilators (e.g., nitroglycerin)
Diuretics (e.g., furosemide)
Cardiotonics (e.g., digoxin)
Cardiac stimulants (e.g., epinephrine, isoproterenol)
Cardiac depressants (e.g., amiodarone)
Antilipidemic drugs (e.g., atorvastatin)
Describe the treatment for MI.
What diagnostic tests usually confirm an MI?
Nursing care of the patient with MI is directed toward detecting complications, preventing further myocardial damage, and promoting comfort, rest, and emotional well-being. Discuss the specific care needs for each situation listed below:
On admission to the CCU
During episodes of chest pain
Fluid retention
Rest
Elimination
Exercise and immobility
Psychological stress
Patient teaching and discharge planning for a cardiac rehabilitation program
Psychological support is imperative for the well-being of the patient with MI. Discuss the patient's potential anxieties and fears and the best means to provide realistic emotional support and reassurance.
Should Patient A make specific lifestyle changes? If so, what changes and how can these be encouraged?
Define silent MI. How common is it?
Patient B is 42 years of age and works as a newspaper editor. He presents to the emergency department complaining of chest pain radiating into both arms, accompanied by diaphoresis and shortness of breath. He has been having episodes of transient substernal and shoulder pain over the past week. He is admitted to the CCU.
Patient B is being treated for hypertension and is currently taking 100 mg metoprolol twice per day. He does not exercise and has smoked a pack of cigarettes daily for 20 years. He reports being under considerable job stress. He is overweight, with a body mass index of 35.
Upon admittance to the CCU, a full physical exam is conducted (Table 5). An ECG shows ST segment depression and T wave inversion consistent with subendocardial ischemia in the inferior and anterior leads. An incomplete left bundle branch block is also noted. Laboratory studies (CBC, urinalysis, and cardiac isoenzyme levels) are all within normal limits, although cardiac isoenzymes are in the upper range.
PATIENT B'S PHYSICAL EXAM RESULTS
| Parameter | Findings | |||
|---|---|---|---|---|
| General appearance |
| |||
| Head and eyes |
| |||
| Ears | Tympanic membranes intact | |||
| Neck |
| |||
| Chest | Symmetrical and clear to auscultation and percussion | |||
| Abdomen |
| |||
| Back | Straight, no costovertebral angle tenderness | |||
| Extremities |
| |||
| Genitourinary system |
| |||
| Neurologic status | Grossly intact | |||
| Cardiovascular system |
| |||
| Vital Signs | ||||
| Blood pressure | 180/100 mm Hg | |||
| Temperature | 98.6° F | |||
| Heart rate | 95 bpm | |||
| Respiratory rate | 20 breaths per minute | |||
Based on the results of the assessment, Patient B is diagnosed with:
Angina pectoris
Subendocardial ischemia
Patient B stays in the CCU for three days. During that time, serum cardiac enzyme levels and repeat ECGs confirm a diagnosis of subendocardial ischemia rather than MI. Coronary artery angiography is done to clarify the coronary artery anatomy and finds a 35% to 45% occlusion of the left anterior descending artery. The possibility of coronary artery vasospasm is not excluded because no ergonovine trial is done. Repeat evaluation for coronary artery bypass surgery is planned for the future, with conservative medical treatment in the interim.
At discharge, Patient B is prescribed:
Digoxin (Lanoxin): 0.25 mg daily
Controlled-release nitroglycerin: 6.5 mg every 12 hours
Nifedipine (Procardia): 10 mg three times daily
Sublingual nitroglycerin (Nitrostat): 0.4 mg as needed for chest pain
Distinguish between the symptoms of angina and MI.
What are the signs and symptoms of stable angina?
Define unstable angina. How is it diagnosed and treated?
Describe Prinzmetal (variant) angina.
What clues suggest the common noncardiac causes of chest pain?
List specific nursing measures regarding medications, diet, activity, lifestyle changes, and emotional support that should be implemented for Patient B.
During his stay in the CCU, Patient B asks if he has to change his lifestyle, as he really did not have a "heart attack." How would you respond?
Discuss the nursing diagnosis of self-concept in regard to patients with angina. How does this major problem impact their perception of self? Their relationships with others?
Patient C, 44 years of age, is brought to the emergency department by ambulance after collapsing at an airport prior to departing on a business trip. He had eaten a large lunch before going to the airport. During the assessment and initiation of treatment, the patient is anxious to return to work. After several short bursts of ventricular tachycardia cause him to become nauseated and short of breath, Patient C agrees to be admitted to the CCU until he feels better.
About three years ago, Patient C noted chest discomfort unrelated to exertion. He tried without success to relieve the chest discomfort with various over-the-counter antacids. Eventually, the pain subsided and he dismissed it with various rationalizations. Two years ago, an ECG done during a routine physical exam was interpreted as normal. This is his first hospital admission.
Family history is positive for early CHD among the men and type 2 diabetes among the women. There are no family members with renal disease, tuberculosis, or cancer.
Patient C is a vice president for a large advertising agency. His job involves frequent travel and entertainment of clients, and he reports frequently drinking alcohol as part of "doing business." This usually consists of a martini at lunch and two whiskey sours before dinner. He smokes occasionally, especially while working on important business deals. He engages in no regular exercise program but does play racquetball occasionally as part of his business-related social life. He owns a home in an affluent neighborhood with his wife; their lifestyle includes entertaining at home and at their country club. Their three teenaged children attend private schools.
Upon admittance to the CCU, a full physical exam is conducted (Table 6). ECG shows sinus tachycardia with frequent PVCs. The atrial and ventricular rate is 114 bpm, and ST segment elevation and depression are noted. Extensive laboratory studies find:
Blood chemistry levels:
Sodium: 140 mEq/L
Potassium: 4.3 mEq/L
Calcium: 109 mEq/L
Carbon dioxide: 23 mEq/L
Blood glucose: 112 mg/dL
Blood urea nitrogen: 17 mEq/L
Uric acid: 6.1 mEq/L
LDH: 237 IU/L
Gamma-glutamyltransferase 1: 26 IU/L
SGOT: 25 IU/L
CK:
Total: 685 IU/dL
CK-MM: 529 IU/L
CK-MB 126 IU/L
Total bilirubin: 0.5 mEq/L
Total cholesterol: 220 mEq/L
Hematology:
Red blood cell: 4.84 cells/mcL
Hemoglobin: 16.4 g/dL
Hematocrit: 47.2%
Mean corpuscular volume: 97.5 fL
Mean cell hemoglobin: 34.0 pg
Mean cell hemoglobin concentration: 34.8%
White blood cell count: 5.1 x 109 cells/L
PATIENT C'S PHYSICAL EXAM RESULTS
| Parameter | Findings | |||
|---|---|---|---|---|
| General appearance |
| |||
| Skin | Moist, cool, dusky | |||
| Head and eyes | Normal | |||
| Ears | Tympanic membranes intact | |||
| Neck |
| |||
| Chest |
| |||
| Abdomen |
| |||
| Extremities | Pulses present and equally moderate in upper extremities and femoral arteries, faint in lower extremities below the groin | |||
| Genitourinary system |
| |||
| Neurologic status |
| |||
| Cardiovascular system |
| |||
| Vital Signs | ||||
| Blood pressure | 98/62 mm Hg | |||
| Temperature | 98.6° F | |||
| Heart rate | 90 bpm with regular irregular rhythm | |||
| Respiratory rate | 24 breaths per minute | |||
A second set of serum enzymes shows an LDH of 298 IU/L and a SGOT of 192 IU/L. Urinalysis reveals straw-colored urine with specific gravity of 1.009, pH of 6, and rare white blood cells per high power field. Patient C's ABGs are also assessed (Table 7).
Based on the results of the assessment, Patient C is diagnosed with acute anterior and inferior MI with early carcinogenic shock.
In the emergency department, oxygen is administered at 4 L/minute via a nasal cannula. Patient C is given lidocaine, 100 mg, as a bolus IV; a lidocaine infusion is started at 2 mg/minute. On arrival in the CCU, the patient is noted to have frequent PVCs as well as one period of five ectopic ventricular beats. A 0.5 mg/kg bolus of IV lidocaine is given, and the infusion rate is increased to 4 mg/minute. The nurse instructs Patient C to notify them if he develops numbness or tingling, chest pain, light-headedness, or other discomfort. A portable chest x-ray is done shortly after he arrives in the CCU and shows pulmonary vascular congestion. IV furosemide (Lasix), 20 mg, is administered, and an indwelling urinary catheter is inserted and connected to a urinometer.
One hour after his arrival in the CCU, Patient C's blood pressure is noted to be barely audible at 60/35 mm Hg. An arterial line is placed in the left radial artery, and a pulmonary artery thermodilution catheter is placed via the left subclavian artery.
An infusion of dopamine hydrochloride (400 mg in 500 mL D5W) is begun at 5 mg/kg/minute. Morphine sulfate is titrated intravenously to reduce the patient's pain, anxiety, and dyspnea. Patient C continues to have 10 to 15 PVCs per minute despite the lidocaine infusion continuing at 4 mg/minute. The oxygen is changed to 15 L/minute by mask. Sodium nitroprusside is cautiously administered as an IV infusion of 50 mg in 250 mL D5W at 0.5 mcg/kg/minute. The patient's blood pressure and cardiac output begin to improve.
Identify and list Patient C's risk factors for developing atherosclerosis.
Define the etiology, pathology, clinical manifestations, and therapeutic treatment of carcinogenic shock. What clinical clues in this case suggest cardiogenic shock?
Explain the rationale for the use of dobutamine, dopamine, and norepinephrine to support blood pressure in the management of shock.
What nursing outcomes would be desirable for Patient C?
What interventions are needed to accomplish these outcomes?
What interventions would be appropriate if Patient C continues to state that he wishes to leave the hospital?
Patient D, 73 years of age, has a history of severe CHD and is admitted to the hospital with a chief complaint of increasing difficulty with angina pectoris that is not controlled with her current medications.
Six years ago, Patient D had a coronary artery bypass procedure. In the past two years, she has been admitted to the hospital several times with unstable angina. Angiograms were done five years, three years, and one month previously.
Coronary angiography one month ago demonstrated complete occlusion of the main stem of the left coronary artery, with previous grafts to the left anterior descending and circumflex branches; partial occlusion of the left anterior descending graft; complete occlusion of the circumflex graft; complete occlusion of the obtuse marginal branch; and partial occlusion of the right coronary artery. The total occlusion of the obtuse marginal branch and partial occlusion of the right coronary artery had developed since the previous angiograms. Following the most recent angiogram, Patient D experienced a significant hypertensive episode that was successfully treated with dopamine hydrochloride infusion, verapamil, and nitroglycerin ointment.
Patient D's father died at 70 years of age of CHD, and her mother had a history of hypertension and died at 91 years of age following a stroke. She has two brothers, one who died at 60 years of age of CHD and diabetes and another brother (70 years of age) with peripheral vascular disease requiring lower extremity vascular bypass surgery. Patient D does not smoke and drinks very little alcohol.
Upon admittance to the CCU, a full physical exam is conducted (Table 8). An ECG shows changes consistent with old anteroseptal and inferior infarcts as well as lateral ischemia. Laboratory studies (CBC, urinalysis, and cardiac isoenzyme levels) are all within normal limits.
PATIENT D'S PHYSICAL EXAM RESULTS
| Parameter | Findings | |||||
|---|---|---|---|---|---|---|
| General appearance |
| |||||
| Head and eyes |
| |||||
| Ears | Unremarkable | |||||
| Neck |
| |||||
| Chest |
| |||||
| Abdomen |
| |||||
| Extremities |
| |||||
| Genitourinary system |
| |||||
| Neurologic status |
| |||||
| Cardiovascular system |
| |||||
| Vital Signs | ||||||
| Blood pressure | 140/70 mm Hg | |||||
| Temperature | 98.6° F | |||||
| Heart rate | 76 bpm with regular rhythm | |||||
| Respiratory rate | 20 breaths per minute | |||||
Based on the results of the assessment, Patient D is diagnosed with unstable angina pectoris, known severe CHD (status post-MI), and status post-coronary artery bypass graft surgery, with one graft clotted.
On the day of admission, Patient D's physician orders a propranolol regimen in an effort to control her anginal pain. This is unsuccessful, and after several days, the physician recommends coronary artery bypass graft surgery. Patient D discusses the proposed surgery with her family and agrees it is necessary. She is scheduled for surgery the next day.
During the procedure, the surgeon places grafts from the aorta to the obtuse marginal and circumflex branches of the left coronary artery as well as to the right coronary artery. Following surgery, the patient has an uncomplicated recovery.
She is returned to the CCU with an endotracheal tube and on a continuous mechanical ventilator. She is weaned from the ventilator slowly and extubated the morning of the first postoperative day. However, Patient D is reluctant to turn, deep breathe, or cough. The nurse tries to ensure adequate pain relief before carrying out these postoperative routines and provides encouragement and support. Family members are allowed to help the patient because she coughs better with their help.
Patient D recovers steadily, but due to her debilitation prior to surgery, her progress is slow. She is transferred out of the CCU on the fifth postoperative day.
What are possible complications of coronary artery bypass graft surgery?
Outline a nursing care plan for Patient D's first two postoperative days.
What information should Patient D and her family receive prior to surgery?
What psychological support measures will be necessary for Patient D postoperatively?
Patient E is a man, 65 years of age, who presents to the emergency department with a two-day history of high-grade fever with chills. He tells the nurse that he does not feel well and believes he may have the flu. He also complains of "some painful bumps" that appeared on his fingers and toes last night. The patient denies any pain other than the lesions on his fingers and toes. He also denies cough, chest pain, breathing problems, palmar or plantar rashes, and vision problems. He does display mild malaise and some loss of appetite.
Patient E reports having had an infected tooth removed about two weeks ago but does not recall taking any antibiotics prior to or after the procedure. He has a history of asthma since childhood and rheumatic fever twice as a child, with mitral valve replacement two years ago. He was diagnosed with hypertension 20 years previously, type 2 diabetes nine years previously, and chronic obstructive pulmonary disease four years previously. Patient E has a 45 pack-year smoking history, but quit when he was diagnosed with emphysema. He also has a history of alcohol abuse, but quit drinking four years ago and continues to attend Alcoholics Anonymous meetings regularly and is active in his church as an usher and frequent volunteer. He denies IV drug abuse.
The patient's mother died following a stroke at 59 years of age and had ovarian cancer. His father had a history of alcohol abuse and type 2 diabetes and suffered an MI at 54 years of age. He died in his 60s from metastatic pancreatic cancer.
Patient E was married for 43 years and is recently widowed and lives alone. He is the father of four and grandfather of 10. One son lives in the same city, but his other children live in other states. He worked as an insurance salesman before retiring last year. Since then, his monthly income has been derived from Social Security, a retirement account, and a small life insurance benefit following his wife's death. He manages his own medications, has no health insurance, and pays for his medications himself.
He reports an allergy to penicillin. As a result of his diagnoses, Patient E is currently taking several medications:
Theophylline: 199 mg twice daily
Albuterol: Two puffs metered-dose inhaler as needed
Ipratropium bromide: Two puffs metered-dose inhaler twice daily
Nadolol: 40 mg once per day
Furosemide: 20 mg once per day
Metformin: 850 mg twice daily
Upon admittance to the CCU, a full physical exam is conducted (Table 9). An ECG is ordered, and the results are normal. Transthoracic echocardiogram indicates 3-cm vegetation on the aortic valve, but no signs of ventricular hypertrophy or dilation. Several laboratory tests are ordered, the results of which are:
Blood chemistry levels:
Sodium: 135 mEq/L
Potassium: 3.7 mEq/L
Chloride: 100 mEq/L
Sodium bicarbonate: 22 mEq/L
Calcium: 8.9 mEq/L
Blood urea nitrogen: 17 mEq/L
Hemoglobin: 14.1 g/dL
Hematocrit: 40%
Platelets: 213,000/mm3
White blood cells: 19,500/mm3
Neutrophils: 80%
Bands: 7%
Lymphocytes: 12%
Monocytes: 1%
Erythrocyte sedimentation rate: 30 mm/hour
PATIENT E'S PHYSICAL EXAM RESULTS
| Parameter | Findings | |||||
|---|---|---|---|---|---|---|
| General appearance |
| |||||
| Skin and nails |
| |||||
| Head and nose |
| |||||
| Eyes |
| |||||
| Ears | Tympanic membranes intact | |||||
| Neck |
| |||||
| Chest |
| |||||
| Abdomen |
| |||||
| Extremities | No cyanosis, clubbing, or edema | |||||
| Genitourinary system |
| |||||
| Neurologic status |
| |||||
| Cardiovascular system |
| |||||
| Vital Signs | ||||||
| Blood pressure | 150/92 mm Hg | |||||
| Temperature | 102.5° F | |||||
| Heart rate | 118 bpm with regular rhythm | |||||
| Respiratory rate | 23 breaths per minute | |||||
Urinalysis reveals pale yellow, clear urine that is negative for proteinuria and hematuria. A urine toxicology screen was also negative. Three blood cultures obtained over one day are positive for Streptococcus viridans. Based on the results of the assessment, Patient E is diagnosed with infective endocarditis, likely originating from the dental infection and procedure.
Which type of infective endocarditis is suggested by Patient E's clinical manifestations—acute or subacute?
Which of the illnesses in Patient E's medical history may be contributing to the onset of infective endocarditis and why are these diseases considered risk factors?
Describe the two clinical types of endocarditis. What are the causative organisms?
How is endocarditis diagnosed and treated?
What are the classic signs and symptoms of endocarditis?
What elements of Patient E's history point to endocarditis?
What are the recommendations for endocarditis prophylaxis?
What is the most significant and relevant clinical finding in Patient E's physical examination so far and what is the patho-physiology that explains this clinical sign?
Identify elevated laboratory test results that are consistent with a diagnosis of bacterial endocarditis.
Explain the pathophysiology for the elevated laboratory results.
Identify subnormal laboratory results that are consistent with a diagnosis of bacterial endocarditis.
What is the significance of the absence of evidence of IV drug abuse?
With knowledge of cardiovascular structures and function and the dynamic pathology that intrudes and impedes normal function, nurses can readily provide quality and often life-saving actions. An awareness of why symptoms appear leads to quicker reporting to physicians of changes in the patient's condition. Nurses can also perform immediate interventions based on standing orders and recognition of what needs to be done in order to provide safe quality care. This knowledge changes what could be only technical care to professional care through the use of decision-making skills built upon the knowledge of pathophysiology.
1. Nowak T, Handford AG. Pathophysiology: Concepts and Applications for Health Care Professionals. 3rd ed. New York, NY: McGraw Hill; 2003.
2. Hall JE. Guyton and Hall Textbook of Medical Physiology (Guyton Physiology). 14th ed. Philadelphia, PA: WB Saunders; 2021.
3. Cerwin BA. Handbook of Pathophysiology. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2014.
5. Price SA. Pathophysiology: Clinical Concepts of Disease Processes. 7th ed. St. Louis, MO: Mosby; 2014.
6. Siezak J. Cellular Interactions in Cardiac Pathophysiology: Developments in Molecular and Cellular Biochemistry. New York, NY: Springer; 2012.
9. Kern MJ, Lim MJ, Goldstein JA. Hemodynamic Rounds: Interpretation of Cardiac Pathophysiology from Pressure Waveform Analysis. 4th ed. Hoboken, NJ: Wiley-Blackwell; 2018.
10. Bullock BA. Focus on Pathophysiology. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2010.
11. McCance KL, Huether SE. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 8th ed. Maryland Heights, MO: Mosby; 2018.
12. Copstead-Kirkhorn LEC, Banasik JL. Pathophysiology. 5th ed. St. Louis, MO: Elsevier Health Sciences; 2013.
13. Brashers VL. Clinical Applications of Pathophysiology: Assessment, Diagnostic Reasoning and Management. 2nd ed. St. Louis, MO: Mosby; 2012.
14. Kupper N, Pedersen SS, Höfer S, Saner H, Oldridge N, Denollet J. Cross-cultural analysis of type D (distressed) personality in 6222 patients with ischemic heart disease: a study from the International HeartQoL Project. Int J Cardiol. 2013;166(2):327-333.
15. Nelms M, Sucher KP. Nutrition Therapy and Pathophysiology. 3rd ed. Belmont, CA: Cenage Learning; 2010.
16. Naeije R. Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis. 2010;52(6):456-466.
18. Black JM, Hawks JH. Medical Surgical Nursing: Clinical Management for Positive Outcomes. 8th ed. Philadelphia, PA: Elsevier Saunders; 2011.
20. Mathers C, Stevens G, Mascarenhas M. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization; 2009.
21. U.S. Preventive Services Task Force. Hypertension in Adults: Screening. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening. Last accessed October 31, 2024.
22. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934.
23. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
24. U.S. Department of Health and Human Services. Smoking Cessation: A Report of the Surgeon General, 2020. Available at https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf. Last accessed October 31, 2024.
25. American Lung Association. About Freedom from Smoking. Available at https://www.lung.org/stop-smoking/join-freedom-from-smoking/about-freedom-from-smoking.html. Last accessed October 31, 2024.
26. Gassner LA, Dunn S, Piller N. Aerobic exercise and the post myocardial infarction patient: a review of the literature. Heart Lung. 2003;32(4):258-265.
27. Institute of Medicine Food and Nutrition Board. Physical Activity. Available at https://www.nap.edu/read/10490/chapter/14. Last accessed October 31, 2024.
28. Ehrman J, Gordon P, Visich P, Keteylan. Clinical Exercise Physiology. 3rd ed. Champaign, IL: Human Kinetics; 2013.
29. Camargo CA Jr, Stampfer MJ, Glynn RJ, et al. Prospective study of moderate alcohol consumption and risk of peripheral arterial disease in US male physicians. Circulation. 1997;95(3):577-580.
30. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671.
31. O'Neill D, Britton A, Hannah MK, et al. Association of longitudinal alcohol consumption trajectories with coronary heart disease: a meta-analysis of six cohort studies using individual participant data. BMC Med. 2018;16(1):124.
32. Hines LM, Stampfer MJ, Ma J, et al. Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N Engl J Med. 2001;344(8):549-555.
33. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 6th ed. Champaign, IL: Human Kinetics; 2021.
34. Jacob R. Controversial Issues in Cardiac Pathophysiology: Erwin Riesch Symposium. New York, NY: Springer; 2013.
35. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115.
36. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
37. Griffin BP. Manual of Cardiovascular Medicine. 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2018.
39. Berg SM, Bittner EA. Massachusetts General Hospital Review of Critical Care Medicine. Philadelphia, PA: Lippincott Williams and Wilkins: 2014.
40. Galvagno SM. Emergency Pathophysiology: Clinical Applications for Prehospital Care. Jackson WY: Teton NewMedia; 2013.
41. Zane RD, Kosowsky JM. Pocket Emergency Medicine (Pocket Notebook Series). 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2018.
42. Amsterdam E, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. J Am Coll Cardiol. 2014;64(24):e139-228.
43. Rosenbaum LS, Januzzi JL. Moving troponin testing into the 21st century: will greater sensitivity be met with greater sensibility? Cardiovasc Hematol Disord Drug Targets. 2008;8(2):118-126.
44. Markovchick VJ, Bakes KA, Buchanan J, Pons PT. Emergency Medicine Secrets. 6th ed. Philadelphia, PA: Elsevier; 2016.
45. Walraven G. Rapid EKG Interpretation. 7th ed. Upper Saddle River, NJ: Prentice Hall Health; 2011.
46. Johns Hopkins Medicine. Event Monitor. Available at https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/event-monitor. Last accessed November 1, 2024.
47. Walsh JA III, Topol EJ, Steinhubl SR. Novel wireless devices for cardiac monitoring. Circulation. 2014;130(7):573-581.
48. Lau JK, Lowres N, Neubeck L, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165(1):193-194.
49. Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(1):95.e11-7.
50. Engel JM, Mehta V, Fororos R, Chavan A. Study of arrhythmia prevalence in NUVANT Mobile Cardiac Telemetry system patients. Conf Proc IEEE Eng Med Biol Soc. 2012;2440-2443.
51. Iftikhar Z, Lahdenoja O, Jafari TM, et al. Multiclass classifier based cardiovascular condition detection using smartphone mechanocardiography. Sci Rep. 2018;8(1):9344.
52. McManus DD, Chong JW, Soni A, et al. PULSE-SMART: pulse-based arrhythmia discrimination using a novel smartphone application. J Cardiovasc Electrophysiol. 2016;27(1):51-57.
53. Garabelli P, Stavrakis S, Po S. Smartphone-based arrhythmia monitoring. Curr Opin Cardiol. 2017;32(1):53-57.
54. American Association of Critical-Care Nurses. AACN Updates Cardiac Monitoring Practice Alerts. Available at https://www.aacn.org/newsroom/aacn-updates-cardiac-monitoring-practice-alerts. Last accessed October 31, 2024.
55. Perrino AC, Reeves ST. A Practical Approach to Transesophageal Echocardiography. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2013.
56. Akinpelu D. Pharmacologic Stress Testing. Available at https://emedicine.medscape.com/article/1827166-overview. Last accessed November 1, 2024.
57. Kern MJ. Cardiac Catheterization Handbook Expert Consult-Online and Print. 5th ed. Philadelphia, PA: Elsevier Saunders; 2011.
58. Kern MJ, Sorajja P, Lim MJ. The Cardiac Catheterization Handbook. 6th ed. Philadelphia, PA: Elsevier Saunders; 2015.
61. Domino FJ. The 5-Minute Clinical Consult Standard 2015: 30-Day Enhanced Online Access + Print (The 5-Minute Consult Series). 23rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2014.
62. DiPiro J, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. Boston, MA: McGraw Hill; 2016.
63. Zerwekh J, Claborn JC. Mosby's Pharmacology Memory Note Cards: Visual, Mnemonic, and Memory Aids for Nurses. 4th ed. Philadelphia, PA: Mosby; 2014.
64. LeMone PT, Burke KM. Medical Surgical Nursing: Critical Thinking in Client Care. 4th ed. Upper Saddle River, NJ: Prentice Hall; 2013.
65. Lilly LS. Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty. 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2010.
66. Ford SM. Roach's Introductory Clinical Pharmacology. 11th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2017.
67. Khan GM. Cardiac Drug Therapy (Contemporary Cardiology). 8th ed. New York, NY: Humana Press; 2015.
68. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425.
69. Simons M, Alpert JS. Acute Coronary Syndrome: Terminology and Classification. Available at https://www.uptodate.com/contents/acute-coronary-syndrome-terminology-and-classification. Last accessed November 1, 2024.
70. Shah A, Gandhi D, Srivastava S, Shah KJ, Mansukhani R. Heart failure: a class review of pharmacotherapy. P T. 2017;42(7):464-472.
72. Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL (eds). Heart failure. In: Braunwald E, Fauci AS, Kasper DL (eds). Harrison's Principles of Internal Medicine. 15th ed. New York, NY: McGraw-Hill; 2011.
73. Gulanick M, Myers JL. Nursing Care Plans: Diagnoses, Interventions, and Outcomes. 9th ed. Philadelphia, PA: Mosby; 2016.
74. Ackley BJ, Ladwig GB, Flynn-Makic MB. Nursing Diagnosis Handbook: An Evidence-Based Guide to Planning Care. 11th ed. Philadelphia, PA: Mosby; 2016.
75. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2016;134(13):e282-e293.
77. Zipes, DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death). J Am Coll Cardiol. 2006;48(5):e247-346.
78. Ferrell BR, Coyle N, Paice J (eds). Oxford Textbook of Palliative Nursing. 4th ed. New York, NY: Oxford University Press; 2015.
79. Hayes DL. Cardiac Pacing, Defibrillation and Resynchronization: A Clinical Approach. 3rd ed. Hoboken, NJ: Wiley-Blackwell; 2013.
80. Mitchell R, Kumar V, Abbas AK, et al. Pocket Companion to Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier Saunders; 2017.
81. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541-1551.
1. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38): 3720-3826. Available at https://academic.oup.com/eurheartj/article/44/38/3720/7243210. Last accessed November 14, 2024.
2. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Card Fail. 2022;28(5):e1-e167. Available at https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063. Last accessed November 14, 2024.
3. AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs, Pettersson GB, Coselli JS, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg. 2017;153(6):1241-1258. Available at https://www.jtcvs.org/article/S0022-5223(17)30116-2/fulltext. Last accessed November 14, 2024.
Mention of commercial products does not indicate endorsement.