Because pain is frequently encountered in the palliative and hospice care environments, a knowledge of appropriate diagnosis and alleviation is vital to all members of the interdisciplinary team. A comprehensive discussion of these topics will provide the knowledge base necessary for all members of the interprofessional team to better understand the varied needs of their patients during the end-of-life period and to be better equipped to address those needs.
This course is designed for physicians, physician assistants, nurses, social workers, and other members of the healthcare team seeking to enhance their knowledge of pain management.
Because pain is frequently encountered in the palliative and hospice care environments, a knowledge of appropriate diagnosis and alleviation is vital to all members of the interdisciplinary team. The purpose of this course is to provide an overview of the assessment and management of pain in the end of life, focusing on the components integral to providing optimum care.
Upon completion of this course, you should be able to:
- Describe the etiology of pain at the end of life and issues in effective pain management.
- Assess pain accurately through use of clinical tools and other strategies, including the use of an interpreter.
- Select appropriate pharmacologic and/or nonpharmacologic therapies to manage pain in patients during the end-of-life period.
John M. Leonard, MD, Professor of Medicine Emeritus, Vanderbilt University School of Medicine, completed his post-graduate clinical training at the Yale and Vanderbilt University Medical Centers before joining the Vanderbilt faculty in 1974. He is a clinician-educator and for many years served as director of residency training and student educational programs for the Vanderbilt University Department of Medicine. Over a career span of 40 years, Dr. Leonard conducted an active practice of general internal medicine and an inpatient consulting practice of infectious diseases.
Contributing faculty, John M. Leonard, MD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Alice Yick Flanagan, PhD, MSW
Margaret Donohue, PhD
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#77144: Assessment and Management of Pain at the End of Life
Unrelieved pain is the greatest fear among people with a life-limiting disease, and the need for an increased understanding of effective pain management is well-documented[1]. Although experts have noted that 75% to 90% of end-of-life pain can be managed effectively, rates of pain are high, even among people receiving palliative care [1,2,3,4,5,6,7,8,9,10].
The inadequate management of pain is the result of several factors related to both patients and clinicians. In a survey of oncologists, patient reluctance to take opioids or to report pain were two of the most important barriers to effective pain relief [11]. This reluctance is related to a variety of attitudes and beliefs [1,11]:
Fear of addiction to opioids
Worry that if pain is treated early, there will be no options for treatment of future pain
Anxiety about unpleasant side effects from pain medications
Fear that increasing pain means that the disease is getting worse
Desire to be a "good" patient
Concern about the high cost of medications
Education and open communication are the keys to overcoming these barriers. Every member of the healthcare team should reinforce accurate information about pain management with patients and families. The clinician should initiate conversations about pain management, especially regarding the use of opioids, as few patients will raise the issue themselves or even express their concerns unless they are specifically asked [12]. It is important to acknowledge patients' fears individually and provide information to help them differentiate fact from fiction. For example, when discussing opioids with a patient who fears addiction, the clinician should explain that the risk of addiction is low [1]. It is also helpful to note the difference between addiction and physical dependence.
There are several other ways clinicians can allay patients' fears about pain medication:
Assure patients that the availability of pain relievers cannot be exhausted; there will always be medications if pain becomes more severe.
Acknowledge that side effects may occur but emphasize that they can be managed promptly and safely and that some side effects will abate over time.
Explain that pain and severity of disease are not necessarily related.
Encouraging patients to be honest about pain and other symptoms is also vital. Clinicians should ensure that patients understand that pain is multidimensional and emphasize the importance of talking to a member of the healthcare team about possible causes of pain, such as emotional or spiritual distress. The healthcare team and patient should explore psychosocial and cultural factors that may affect self-reporting of pain, such as concern about the cost of medication.
Clinicians' attitudes, beliefs, and experiences also influence pain management, with addiction, tolerance, side effects, and regulations being the most important concerns [1,8,11,13,14,15]. A lack of appropriate education and training in the assessment and management of pain has been noted to be a substantial contributor to ineffective pain management [11,13,15,16]. As a result, many clinicians, especially primary care physicians, do not feel confident about their ability to manage pain in their patients [11,13].
Cultural and demographic factors may also contribute to lack of effective pain management. Expression of pain and the use of pain medication differ across cultures. For example, Hispanic and Filipino patients have been shown to be reluctant to report pain because of fear of side effects or addiction [17]. Even when effective opioids have been prescribed, access may be difficult, as inadequate supplies of opioids are more likely in pharmacies in primarily nonwhite neighborhoods [18]. Communication with patients regarding level of pain is a vital aspect of caring for patients in the end of life. When there is an obvious disconnect in the communication process between the practitioner and patient due to the patient's lack of proficiency in the English language, an interpreter is required.
The prevalence of pain at the end of life has been reported to range from 8% to 96%, occurring at higher rates among people with cancer than among adults with other life-limiting diseases [19,20]. Pain can be caused by a multitude of factors and is usually multidimensional, with pain frequently being exacerbated by other physical symptoms and by psychosocial factors, such as anxiety or depression [8].
Pain should be assessed routinely, and frequent assessment has become the standard of care [8]. Pain is a subjective experience, and as such, the patient's self-report of pain is the most reliable indicator. Research has shown that pain is underestimated by healthcare professionals and overestimated by family members [8,21]. Therefore, it is essential to obtain a pain history directly from the patient, when possible, as a first step toward determining the cause of the pain and selecting appropriate treatment strategies. When the patient is unable to communicate verbally, other strategies must be used to determine the characteristics of the pain, as will be discussed.
Questions should be asked to elicit descriptions of the pain characteristics, including its location, distribution, quality, temporal aspect, and intensity. In addition, the patient should be asked about aggravating or alleviating factors. Pain is often felt in more than one area, and physicians should attempt to discern if the pain is focal, multifocal, or generalized. Focal or multifocal pain usually indicates an underlying tissue injury or lesion, whereas generalized pain could be associated with damage to the central nervous system. Pain can also be referred, usually an indicator of visceral pain.
The quality of the pain refers to the sensation experienced by the patient, and it often suggests the pathophysiology of the pain [8]. Pain that is well localized and described as aching, throbbing, sharp, or pressure-like is most likely somatic nociceptive pain. This type of pain is usually related to damage to bones and soft tissues. Diffuse pain that is described as squeezing, cramping, or gnawing is usually visceral nociceptive pain. Pain that is described as burning, tingling, shooting, or shock-like is neuropathic pain, which is generally a result of a lesion affecting the nervous system.
Temporal aspects of pain refer to its onset: acute, chronic, or "breakthrough." A recent onset characterizes acute pain, and there are accompanying signs of generalized hyperactivity of the sympathetic nervous system (diaphoresis and increased blood pressure and heart rate). Acute pain usually has an identifiable, precipitating cause, and appropriate treatment with analgesic agents will relieve the pain. When acute pain develops over several days with increasing intensity, it is said to be subacute. Episodic, or intermittent, pain occurs during defined periods of time, on a regular or irregular basis. Chronic pain is defined as pain that persists for at least three months beyond the usual course of an acute illness or injury. Such pain is not accompanied by overt pain behaviors (grimacing, moaning) or evidence of sympathetic hyperactivity.
"Breakthrough" is the term used to describe transitory exacerbations of severe pain over a baseline of moderate pain [22]. Breakthrough pain can be incident pain or pain that is precipitated by a voluntary act (such as movement or coughing) or can occur without a precipitating event. Breakthrough pain occurs in as many as 90% of people with cancer or in hospice settings and is often a consequence of inadequate pain management [1].
Documentation of pain intensity is key, as several treatment decisions depend on the intensity of the pain. For example, severe, intense pain requires urgent relief, which affects the choice of drug and the route of administration [8,23]. The numeric rating scale is the tool used most often to assess pain; with this tool, patients rate pain on a scale of 0 to 10 [8]. Visual analogue scales (patients rate pain on a line from 0 to 10) and verbal rating scales, which enable the patient to describe the pain as "mild," "moderate," or "severe," have also been found to be effective. Some patients, however, may have difficulty rating pain using even the simple scales. In an unpublished study involving 11 adults with cancer, the Wong-Baker FACES scale, developed for use in the pediatric setting, was found to be the easiest to use among three pain assessment tools that include faces to assess pain [24].
Functional assessment is important. The healthcare team should observe the patient to see how pain limits movements and should ask the patient or family how the pain interferes with normal activities. Determining functional limitations can help enhance patient compliance in reporting pain and adhering to pain-relieving measures, as clinicians can discuss compliance in terms of achieving established functional goals [12]. The Memorial Pain Assessment Card can be used to evaluate both the severity of pain and the effect of pain on function [8,25].
Physical examination can be valuable in determining an underlying cause of pain. Examination of painful areas can detect evidence of trauma, skin breakdown, or changes in osseous structures. Auscultation can detect abnormal breath or bowel sounds; percussion can detect fluid accumulation; and palpation can reveal tenderness. A neurologic examination should also be carried out to evaluate sensory and/or motor loss and changes in reflexes. During the examination, the clinician should watch closely for nonverbal cues that suggest pain, such as moaning, grimacing, and protective movements. These cues are especially important when examining patients who are unable to verbally communicate about pain.
Strong evidence supports pain management approaches for people with cancer, but the evidence base for management of pain in people with other life-limiting diseases is weak [2,4,26,27,28,29,30]. Effective pain management involves a multidimensional approach involving pharmacologic and nonpharmacologic interventions that are individualized to the patient's specific situation [8].
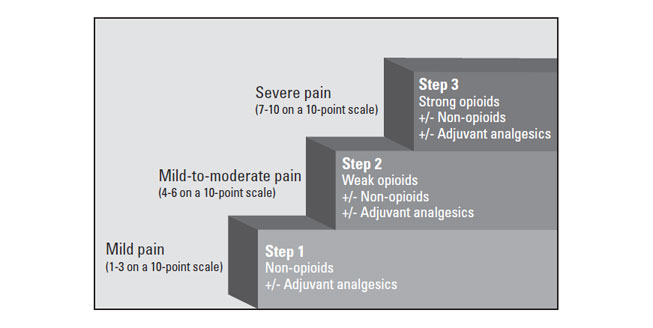
The WHO analgesic ladder, introduced in 1986 and disseminated worldwide, remains recognized as a useful educational tool but not as a strict protocol for the treatment of pain. It is intended to be used only as a general guide to pain management [31]. The three-step analgesic ladder designates the type of analgesic agent based on the severity of pain (Figure 1) [31]. Step 1 of the WHO ladder involves the use of nonopioid analgesics, with or without an adjuvant (co-analgesic) agent, for mild pain (pain that is rated 1 to 3 on a 10-point scale). Step 2 treatment, recommended for moderate pain (score of 4 to 6), calls for a weak opioid, which may be used in combination with a step 1 nonopioid analgesic for unrelieved pain. Step 3 treatment is reserved for severe pain (score of 7 to 10) or pain that persists after Step 2 treatment. Strong opioids are the optimum choice of drug at Step 3. At any step, nonopioids and/or adjuvant drugs may be helpful. Some consider this model to be outdated and/or simplistic, but most agree that it remains foundational. It can be modified or revised, as needed, to apply more accurately to different patient populations.
The WHO ladder is also accompanied by five guiding principles [31]:
Reduce pain to levels that allow an acceptable quality of life.
Global assessment of the patient should guide treatment, recognizing that individuals experience and express pain differently.
The safety of patients, carers, healthcare providers, communities, and society must be assured.
A pain management plan includes pharmacologic treatments and may include psychosocial and spiritual care.
Analgesics, including opioids, must be accessible: both available and affordable.
The pharmacologic treatment of pain involves selecting the right drug(s) at the right dose, frequency, and route, and managing side effects [8].
Nonopioid analgesics, such as aspirin, acetaminophen (Tylenol), and nonsteroidal anti-inflammatory drugs (NSAIDs), are primarily used for mild pain (Step 1 of the WHO ladder) and may also be helpful as coanalgesics at Steps 2 and 3. Acetaminophen is among the safest of analgesic agents, but it has essentially no anti-inflammatory effect. Toxicity is a concern at high doses, and the maximum recommended dose is 3–4 g per day [8]. Acetaminophen should be avoided or given at lower doses in people with a history of alcohol abuse or renal or hepatic insufficiency [8].
NSAIDs are most effective for pain associated with inflammation. Among the commonly used NSAIDs are ibuprofen (Motrin, Advil), naproxen (Aleve, Naprosyn), and indomethacin (Indocin). There are several classes of NSAIDs, and the response differs among patients; trials of drugs for an individual patient may be necessary to determine which drug is most effective [33]. NSAIDs inhibit platelet aggregation, increasing the risk of bleeding, and also can damage the mucosal lining of the stomach, leading to gastrointestinal bleeding. There is a ceiling effect to the nonopioid analgesics; that is, there is a dose beyond which there is no further analgesic effect. In addition, many side effects of nonopioids can be severe and may limit their use or dosing.
Moderate pain (Step 2) has often been treated with analgesic agents that are combinations of acetaminophen and an opioid, such as codeine, oxycodone, or hydrocodone. However, it is now recommended that these combination drugs be avoided, as limits on the maximum dose of acetaminophen limits the use of a combination drug [8,34]. Individual drugs in combination is preferred, allowing for increases in the dose of the opioid without increasing the dose of the co-analgesic.
Strong opioids are used for severe pain (Step 3). Guidelines suggest that the most appropriate opioid dose is the dose required to relieve the patient's pain throughout the dosing interval without causing unmanageable side effects [4,8,26,28,30,34,36]. Morphine, buprenorphine, oxycodone, hydromorphone, fentanyl, and methadone are the most widely used Step 3 opioids in the United States [35]. Unlike nonopioids, opioids do not have a ceiling effect, and the dose can be titrated until pain is relieved or side effects become unmanageable. For an opioid-naïve patient or a patient who has been receiving low doses of a weak opioid, the initial dose of a Step 3 opioid should be low, and, if pain persists, the dose may be titrated up daily until pain is controlled. Opioid-naïve patients are those who are not receiving opioid analgesic daily and therefore have not developed significant tolerance. Opioid-tolerant patients are those who have been taking an opioid analgesic daily for at least one week. The FDA identifies tolerance as receiving at least 60 mg of morphine daily, 30 mg of oral oxycodone daily, 8 mg of oral hydromorphone daily, or an equianalgesic dose of another opioid for one week or longer [30]. Typical starting doses for patients who are opioid-naïve have been noted, but these doses should be used only as a guide, and the initial dose, as well as titrated dosing, should be done on an individual basis (Table 1).
OPIOIDS FOR THE MANAGEMENT OF PAIN IN ADULTSa
| Drug | Typical Starting Doseb | Onset of Action | Duration of Action | ||
|---|---|---|---|---|---|
| Codeine | 15–60 mg | 30 to 60 minutes | 4 to 6 hours | ||
| Hydrocodone | 2.5–10 mg | 10 to 20 minutes | 4 to 8 hours | ||
| Morphine, immediate release | 15–30 mg |
| 3 to 6 hours | ||
| Oxycodone, immediate release | 5–10 mg | 10 to 30 minutes | 3 to 4 hours | ||
| Oxymorphone, sustained release | 10 mg | 5 to 10 minutes | 8 to 12 hours | ||
| Hydromorphone | 2–4 mg | 15 to 30 minutes | 4 to 5 hours | ||
| Methadone | 5–10 mg | 30 to 60 minutes | 4 to 6 hours | ||
| Tapentadol | 50–100 mg | <60 minutes | 4 to 6 hours | ||
| Tapentadol, extended release | 50–100 mg | — | — | ||
| Fentanyl (buccal tablet) | 100–200 mcg | 5 to 15 minutes | 2 to 4 hours | ||
| Fentanyl (transdermal patch) | 25 mcg/hour (worn for 3 days) | 12 to 18 hours | 48 to 72 hours | ||
| Buprenorphine (transdermal patch) | 5–10 mcg/hour (worn for 7 days) | — | — | ||
| |||||
The most serious potential adverse effect following initiation of opioids for treatment of pain is oversedation followed by respiratory depression. To mitigate this risk, clinicians should discuss the role of naloxone administration by caregivers in the event of sedation/respiratory depression and make naloxone available as indicated or as required by local regulations [30]. When initiating morphine, or any opioid agent for treatment of moderate/severe pain, the prescribing clinician should consider lower starting dose titration in frail or older patients and in any patient with renal insufficiency (reduced creatinine clearance).
More than one route of opioid administration will be needed by many patients during end-of-life care, but in general, opioids should be given orally, as this route is the most convenient and least expensive. The transdermal route is preferred to the parenteral route, although dosing with a transdermal patch is less flexible and may not be appropriate for patients with unstable pain [8]. Intramuscular injections should be avoided because injections are painful, drug absorption is unreliable, and the time to peak concentration is long [8].
Morphine is considered to be the first-line treatment for a Step 3 opioid [34]. Morphine is available in both immediate-release and sustained-release forms, and the latter form can enhance patient compliance. The sustained-release tablets should not be cut, crushed, or chewed, as this counteracts the sustained-release properties. Morphine should be avoided in patients with severe renal failure [28].
Buprenorphine (Butrans) has the general structure of morphine but differs from it in several ways [35]. The transdermal formulation of the drug was approved in 2010 for moderate-to-severe chronic pain in patients requiring an around-the-clock opioid for an extended period [8]. It may be used for people with renal impairment but is contraindicated in patients who have substantial respiratory depression [35,37].
The sustained-release form of oxycodone (OxyContin) has been shown to be as safe and effective as morphine for cancer-related pain, and it may be associated with less common side effects, especially hallucinations and delirium [40]. Oxycodone is also available in an immediate-release form (Roxicodone). Oxycodone should be used in people with advanced chronic kidney disease only if alternative options are not available [28]. If the drug must be used, the intervals between doses should be increased, and the patient should be monitored closely [28].
Hydromorphone and fentanyl are the most potent opioids; neither drug should be given to an opioid-naïve patient. Hydromorphone, which is four times as potent as morphine, is available in immediate- and extended-release forms [41]. Fentanyl is the strongest opioid (approximately 80 times the potency of morphine) and is available as a transdermal drug-delivery system (Duragesic; Ionsys); a buccal film (Onsolis) and tablet (Fentora); a nasal spray (Lazanda); a sublingual spray (Subsys); a sublingual tablet (Abstral); and a lozenge (Actiq) [37,42]. Fentanyl preparations have a more rapid onset than other opioids given nonparenterally [8]. Because of its potency, fentanyl must be used with extreme care, as deaths have been associated with its use. Physicians must emphasize to patients and their families the importance of following prescribing information closely, and members of the healthcare team should monitor the use of the drug. Fentanyl, administered subcutaneously, is the recommended choice for patients with advanced chronic kidney disease [28].
The use of methadone to relieve pain has increased substantially over the past few years, moving from a second-line or third-line drug to a first-line medication for severe pain in people with life-limiting diseases [43]. A systematic review showed that methadone had efficacy similar to that of morphine [44]. However, the authors' conclusions were based on low-quality evidence. Other opioids (e.g., morphine, fentanyl) are easier to manage but may be more expensive than methadone in many economies [44]. Physicians must be well educated about the pharmacologic properties of methadone, as the risk for serious adverse events, including death, is high when the drug is not administered appropriately [44,45]. If the dose of methadone is increased too rapidly or administered too frequently, toxic accumulation of the drug can cause respiratory depression and death. Because of the unique nature of methadone, and its long and variable half-life, extreme care must be taken when titrating the drug, and frequent and careful evaluation of the patient is required. Practitioners are advised to consult with a pain or palliative care specialist if they are unfamiliar with methadone prescribing or if individual patient considerations necessitate rapid switching to or from methadone [4].
Meperidine (Demerol) should not be used in the palliative care setting because of limited efficacy and potential for severe toxicity [12,33]. Agonist-antagonist opioids (nalbuphine [Nubain], butorphanol [Stadol], and pentazocine [Talwin]) are not recommended for use with pure opioids, as they compete with them, leading to possible withdrawal symptoms.
Tapentadol (Nucynta) is a short-acting opioid approved for moderate to severe pain in adults; an extended release formulation (Nucynta ER) was approved in 2011 for moderate-to-severe chronic pain when an around-the-clock opioid is needed [46]. The drug is associated with a lower incidence of adverse effects than other opioids, and it has been shown to be highly effective for chronic pain conditions but has not been extensively studied in cancer-related pain or the palliative care setting [47]. A 2014 study of 123 patients that had previously received long-term analgesia for cancer-related pain showed tapentadol significantly reduced pain scores and was generally well tolerated; concomitant use of pain medications was also reduced [48].
The most appropriate option for breakthrough pain is an immediate-release opioid taken in addition to the around-the-clock regimen [8]. The fentanyl buccal tablet has been shown to be effective and safe for relieving breakthrough pain in people who are opioid tolerant [4,49,50]. Between January 2011 and January 2012, three forms of fentanyl were approved for breakthrough pain in people with cancer: fentanyl sublingual tablet (Abstral), fentanyl nasal spray (Lazanda), and fentanyl sublingual spray (Subsys) [37]. Abstral and Lazanda have since been discontinued [37,41]. As of 2021, the fentanyl lozenge (Actiq) and buccal tablet (Fentora) are also approved for breakthrough cancer pain [41]. For each formula, the initial dose may be repeated once if pain is not relieved adequately after 30 minutes. Patients must wait at least two hours before using the sublingual tablet, buccal film, or the nasal spray for another breakthrough pain episode; the interval is four hours for the sublingual spray, lozenge, or buccal tablet [37,41].
When pain responds poorly to escalated doses of an opioid, other approaches should be considered, including alternative routes of administration, use of alternate opioids (termed opioid rotation or opioid switching), use of adjuvant analgesics, and nonpharmacologic approaches. A process for opioid switching has been established; the first step is to calculate the equianalgesic dose of the new drug [4,8,34]. Additional care is needed when switching to methadone, and conversion ratios have been established [4]. Evidence suggests that the traditionally recommended equianalgesic doses for the fentanyl transdermal patch are subtherapeutic for patients with chronic cancer-related pain, and more aggressive approaches may be warranted [4,8,51].
Another approach that has been used for pain management in the cancer setting is combination opioid therapy, or the concurrent use of two strong opioids. The effectiveness of this approach has been evaluated in only two studies, and the combination was morphine and oxycodone or morphine with fentanyl or methadone [52]. The evidence to support a recommendation of combination opioid therapy is weak, and the side effects most likely outweigh the benefit [52].
Opioids are associated with many side effects, the most notable of which is constipation, occurring in nearly 100% of patients. The universality of this side effect mandates that once extended treatment with an opioid begins, prophylactic treatment with laxatives must also be initiated. Tolerance to other side effects, such as nausea and sedation, usually develops within three to seven days. Some patients may state that they are "allergic" to an opioid. It is important for the physician to explore what the patient experienced when the drug was taken in the past, as many patients misinterpret side effects as an allergy. True allergy to an opioid is rare [8]. Opioid rotation may also be done to reduce adverse events.
When opioids are prescribed, careful documentation of the patient's history, examinations, treatments, progress, and plan of care are especially important from a legal perspective. This documentation must provide evidence that the patient is functionally better off with the medication than without [33]. In addition, physicians must note evidence of any dysfunction or abuse.
Adjuvant agents are often used in conjunction with opioids and are usually considered after the use of opioids has been optimized [33]. The primary indication for these drugs is adjunctive because they can provide relief in specific situations, especially neuropathic pain. Examples of adjuvant drugs are tricyclic antidepressants, anticonvulsants, muscle relaxants, and corticosteroids (Table 2) [4,8]. A systematic review found that there was limited evidence to support the use of selective serotonin reuptake inhibitors (SSRIs) for neuropathic pain, but one serotonin-norepinephrine reuptake inhibitor, venlafaxine (Effexor), was found to be effective [53].
ORAL ADJUVANT ANALGESICS
| Drug Class | Drug | Typical Starting Dose | Usual Effective Dose | |||
|---|---|---|---|---|---|---|
| Anticonvulsants | Gabapentin | 100–300 mg once daily | 300–1,200 mg (2 or 3 divided doses) | |||
| Pregabalin | 25–75 mg twice daily | 75–200 mg (3 divided doses) | ||||
| Carbamazepine | 50–100 mg twice daily | 300–600 mg twice daily | ||||
| Topiramate | 25–50 mg daily | 50–200 mg twice daily | ||||
| Oxcarbazepine | 150–300 mg twice daily | 150–600 mg twice daily | ||||
| Tiagabine | 4 mg at bedtime | 4–12 mg twice daily | ||||
| Tricyclic antidepressants |
| 10–25 mg at bedtime | 50–150 mg at bedtime | |||
| Serotonin-norepinephrine reuptake inhibitors | Venlafaxine | 37.5 mg daily | 150–350 mg daily | |||
| Skeletal muscle relaxants | Baclofen | 5 mg twice daily | 10–20 mg 2 or 3 times daily | |||
| Cyclobenzaprine | 5 mg 3 times daily | 10–20 mg 3 times daily | ||||
| Metaxalone | 400 mg 3 times daily | Not defined | ||||
| Corticosteroids | Dexamethasone | 1–2 mg | Not defined |
Several nonpharmacologic approaches are therapeutic complements to pain-relieving medication, lessening the need for higher doses and perhaps minimizing side effects. These interventions can help decrease pain or distress that may be contributing to the pain sensation. Approaches include palliative radiotherapy, complementary/alternative methods, manipulative and body-based methods, and cognitive/behavioral techniques. The choice of a specific nonpharmacologic intervention is based on the patient's preference, which, in turn, is usually based on a successful experience in the past.
Palliative radiotherapy is effective for managing cancer-related pain, especially bone metastases [2,54,55]. Bone metastases are the most frequent cause of cancer-related pain; 50% to 75% of patients with bone metastases will have pain and impaired mobility [54]. External-beam radiotherapy is the mainstay of treatment for pain related to bone metastases. At least some response occurs in 70% to 80% of patients, and the median duration of pain relief has been reported to be 11 to 24 weeks [54]. It takes one to four weeks for optimal therapeutic results [54,55].
However, palliative radiotherapy has become a controversial issue. Although the benefits of palliative radiotherapy are well documented and most hospice and oncology professionals believe that palliative radiotherapy is important, this treatment approach is offered at approximately 24% of Medicare-certified freestanding hospices, with less than 3% of hospice patients being treated [56,57,58]. As previously noted, reimbursement issues present a primary barrier to the use of palliative radiotherapy [56,57,58]. Among other barriers are short life expectancy, transportation issues, patient inconvenience, and lack of knowledge about the benefits of palliative radiotherapy in the primary care community [55,56,57,59].
One study found that more than half (54%) of people use complementary/alternative medicine therapies at the end of life [60]. The most commonly used therapies are massage, music, relaxation techniques, and acupuncture [60,61,62,63,64].
Massage, which can be broadly defined as stroking, compression, or percussion, has led to significant and immediate improvement in pain in the hospice setting [65]. Both massage and vibration are primarily effective for muscle spasms related to tension or nerve injury, and massage can be carried out with simultaneous application of heat or cold. Massage may be harmful for patients with coagulation abnormalities or thrombophlebitis [12].
Focused relaxation and breathing can help decrease pain by easing muscle tension. Progressive muscle relaxation, in which patients follow a sequence of tensing and relaxing muscle groups, has enabled patients to feel more in control and to experience less pain and can also help provide distraction from pain [12]. This technique should be avoided if the muscle tensing will be too painful.
Acupuncture typically provides pain relief 15 to 40 minutes after stimulation. Relief seems to be related to the release of endorphins and a susceptibility to hypnosis [12]. The efficacy of acupuncture for relieving pain has not been proven, as study samples have been small. However, acupuncture has been found to be of some benefit for cancer-related pain when the therapy is given in conjunction with analgesic therapy [66].
Other nonpharmacologic interventions that have been helpful for some patients but lack a strong evidence base include manipulative and body-based methods (such as application of cold or heat, and positioning), yoga, distraction, and music or art therapy. The application of cold and heat are particularly useful for localized pain and have been found to be effective for cancer-related pain caused by bone metastases or nerve involvement, as well as for prevention of breakthrough incident pain [12]. Alternating application of heat and cold can be soothing for some patients, and it is often combined with other nonpharmacologic interventions.
Cold can be applied through wraps, gel packs, ice bags, and menthol. It provides relief for pain related to skeletal muscle spasms induced by nerve injury and inflamed joints. Cold application should not be used for patients with peripheral vascular disease. Heat can be applied as dry (heating pad) or moist (hot wrap, tub of water) and should be applied for no more than 20 minutes at a time, to avoid burning the skin. Heat should not be applied to areas of decreased sensation or with inadequate vascular supply, or for patients with bleeding disorders.
Changing the patient's position in the bed or chair may help relieve pain and also helps minimize complications such as decubitus ulcers, contractures, and frozen joints. Members of the healthcare team as well as family members and other informal caregivers can help reposition the patient for comfort and also perform range-of-motion exercises. Physical and occupational therapists can recommend materials, such as cushions, pillows, mattresses, splints, or support devices.
Hatha yoga is the branch of yoga most often used in the medical context, and it has been shown to provide pain relief for patients who have osteoarthritis and carpal tunnel syndrome but it has not been studied in patients at the end of life. Yoga may help relieve pain indirectly in some patients through its effects on reducing anxiety, increasing strength and flexibility, and enhancing breathing [67]. Yoga also helps patients feel a sense of control.
Methods to provide distraction from pain come in a wide variety of methods, including reciting poetry, meditating with a calm phrase, watching television or movies, playing cards, visiting with friends, or participating in crafts.
Music therapy and art therapy are also becoming more widely used as nonpharmacologic options for pain management. Listening to music has been shown to decrease the intensity of pain and reduce the amount of opioids needed, but the magnitude of the benefit was small [68]. Research suggests that art therapy contributes to a patient's sense of well-being [69]. Creating art helps patients and families to explore thoughts and fears during the end of life. An art therapist can help the creators reflect on the implications of the art work. Art therapy is especially helpful for patients who have difficulty expressing feelings with words, for physical or emotional reasons.
Fear of license suspension for inappropriate prescribing of controlled substances is also prevalent, and a better understanding of pain medication will enable physicians to prescribe accurately, alleviating concern about regulatory oversight. Physicians must balance a fine line; on one side, strict federal regulations regarding the prescription of schedule II opioids (morphine, oxycodone, methadone, hydromorphone) raise fear of Drug Enforcement Agency investigation, criminal charges, and civil lawsuits [1,70]. Careful documentation on the patient's medical record regarding the rationale for opioid treatment is essential [70]. On the other side, clinicians must adhere to the American Medical Association's Code of Ethics, which states that failure to treat pain is unethical. The code states, in part: "Physicians have an obligation to relieve pain and suffering and to promote the dignity and autonomy of dying patients in their care. This includes providing effective palliative treatment even though it may foreseeably hasten death" [71]. In addition, the American Medical Association Statement on End-of-Life Care requires that physicians "reassure the patient and/or surrogate that all other medically appropriate care will be provided, including aggressive palliative care and appropriate symptom management, if that is what the patient wishes"[72].
Physicians should consider the legal ramifications of inadequate pain management and understand the liability risks associated with both inadequate treatment and treatment in excess. The undertreatment of pain carries a risk of malpractice liability, and this risk is set to increase as the general population becomes better educated about the availability of effective approaches to pain management at the end of life. Establishing malpractice requires evidence of breach of duty and proof of injury and damages. Before the development of various guidelines for pain management, it was difficult to establish a breach of duty, as this principle is defined by nonadherence to the standard of care in a designated specialty. With such standards now in existence, expert medical testimony can be used to demonstrate that a practitioner did not meet established standards of care for pain management. Another change in the analysis of malpractice liability involves injury and damages. Because pain management can be considered as separate from disease treatment and because untreated pain can lead to long-term physical and emotional damage, claims can be made for pain and suffering alone, without wrongful death or some other harm to the patient [73].
The proper storage and disposal of prescription pain medications should also be considered. Taking steps to ensure that medications are stored and destroyed securely and safely can help prevent unintended overdose and substance abuse. In 2010, the U.S. Senate passed the Secure and Responsible Drug Disposal Act, which amended the Controlled Substances Act to permit the take-back disposal of medications by authorized persons (rather than the patient with the prescription) [74]. As such, healthcare professionals may be required to dispose of drugs returned by patients in addition to drug samples that have expired or are not being dispensed. For best practice guidelines on the disposal of medications by patients or healthcare professionals, please visit the Drug Enforcement Administration Office of Diversion Control at https://www.deadiversion.usdoj.gov/drug_disposal/drug-disposal.html [32].
This population of people with a history of substance abuse presents challenges to the effective use of pain medication, with issues related to trust, the appropriate use of pain medications, interactions between illicit drugs and treatment, and compliance with treatment. The issues differ depending on whether substance abuse is a current or past behavior.
With active substance abusers, it is difficult to know if patients' self-reports of pain are valid or are drug-seeking behaviors. It has been recommended that, as with other patients at the end of life, self-reports of pain should be believed [12,33]. A multidisciplinary approach, involving psychiatric professionals, addiction specialists, and, perhaps, a pain specialist, is necessary. To decrease the potential for the patient to seek illicit drugs for pain, an appropriate pain management plan should be implemented and the patient should be reassured that pain can be managed effectively [12,33]. When planning treatment, the patient's tolerance must be considered; higher doses may be needed initially, and doses can be reduced once acute pain is under control. Long-acting pain medications are preferred for active substance abusers, and the use of nonopioids and coanalgesics can help minimize the use of opioids. Setting limits as well as realistic goals is essential and requires establishing trust and rapport with the patient and caregivers.
Establishing trust is also essential for patients with former substance abuse behavior, who often must be encouraged to adhere to a pain management program because of their fears of addiction. Involving the patient's drug counselor is beneficial, and other psychological clinicians may be helpful in assuring the patient that pain can be relieved without addiction. Recurrence of addiction is low, especially among people with cancer, but monitoring for signs of renewed abuse should be ongoing [12].
Patients who are following a methadone maintenance program may also fear effective pain management as a risk for recurrent abuse. Two approaches may be followed for these patients: they may receive an increased dose of methadone as the pain reliever or they may be given other opioids along with the same methadone dose, with the dose of the opioid titrated for effective pain relief [12,33]. Again, involvement of the drug counselor is important.
As many as 96% of people with a life-limiting disease have pain at the end of life, and unrelieved pain is a great fear in this population. However, experts estimate that 75% to 90% of end-of-life pain can be effectively managed. Healthcare professionals should strive to enhance their knowledge of key strategies to achieve high-quality pain management at the end of life through open communication, frequent assessment, and the use of evidence-based pharmacologic and nonpharmacologic interventions that are individualized to each patient's specific situation.
1. American Pain Foundation. Breakthrough cancer pain: mending the break in the continuum of care. J Pain and Palliat Care Pharmacother. 2011;25(3):252-264.
2. Qaseem A, Snow V, Shekelle P, et al. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148(2):141-146.
3. Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage. 2010;39(3):477-485.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Palliative Care. Version 1.2024. Available at https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. Last accessed October 16, 2024.
5. Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35(6):594-603.
6. Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38(1):115-123.
7. Esther Kim J-E, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37(4):715-736.
8. Dalal S, Bruera E. Assessment and management of pain in the terminally ill. Prim Care Clin Office Pract. 2011;38:195-223.
9. Gao W, Gulliford M, Higginson IJ. Prescription patterns of analgesics in the last 3 months of life: a retrospective analysis of 10,202 lung cancer patients. Br J Cancer. 2011;104(11):1704-1710.
10. Godfrey C, Harrison MB, Medves J, Tranmer JE. The symptom of pain with heart failure: a systematic review. J Card Fail. 2006;12(4):307-313.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists' attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Abrahm JL. A Physician's Guide to Pain and Symptom Management in Cancer Patients. 3rd ed. Baltimore, MD: Johns Hopkins University Press; 2014.
14. Zerzan J, Lee CA, Haverhals LM, Nowels CT. Exploring physician decisions about end-of-life opiate prescribing: a qualitative study.J Palliat Med. 2011;14(5):567-572.
15. Fineberg IC, Wenger NS, Brown-Saltzman K. Unrestricted opiate administration for pain and suffering at the end of life: knowledge and attitudes as barriers to care. J Palliat Med. 2006;9(4):873-883.
16. Mezei L, Murinson BB, Johns Hopkins Pain Curriculum Development Team. Pain education in North American medical schools.J Pain. 2011;12(12):1199-1208.
17. Steinhauser K, Clipp EC, McNeilly M, et al. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132:825-832.
18. Morrison RS, Wallenstein S, Natale DK, Senzel RS, Huang LL. "We don't carry that:" failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N Engl J Med. 2000;342(14):1023-1026.
19. Solano J, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58-69.
20. Goudas LC, Bloch R, Gialeli-Goudas M, Lau J, Carr DB. The epidemiology of cancer pain. Cancer Invest. 2005;23:182-190.
21. Desbiens NA, Mueller-Rizner N. How well do surrogates assess the pain of seriously ill patients? Crit Care Med. 2000;28(5):1347-1352.
22. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273-281.
23. World Health Organization. Cancer Pain Relief and Palliative Care. Technical Report Series 804. Geneva: World Health Organization; 1990.
24. Hockenberry M, Wilson D. Wong's Essentials of Pediatric Nursing. 9th ed. St. Louis, MO: Mosby; 2013.
25. Fishman B, Pasteranak S, Wallenstien SL, Houde RW, Holland JC, Foley KM. The Memorial Pain Assessment Card: a valid instrument for the evaluation of cancer pain. Cancer. 1987;60:1151-1158.
26. Adler ED, Goldfinger JZ, Park ME, Meier DE. Palliative care in the treatment of advanced heart failure. Circulation. 2009;120:2597-2606.
27. Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med. 2008;148(2):147-159.
28. Douglas C, Murtagh FE, Chambers EJ, Howse M, Ellershaw J. Symptom management for the adult patient dying with advanced chronic kidney disease: a review of the literature and development of evidence-based guidelines by a United Kingdom Expert Consensus Group. Palliat Med. 2009;23(2):103-110.
29. Ford DW, Koch KA, Ray DE, Selecky PA. Palliative and end-of-life care in lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e498S-e512S.
30. National Comprehensive Cancer Network. NCCN Guidelines Version 2.2024 Adult Cancer Pain. Available at https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Last accessed October 14, 2024.
31. World Health Organization. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Geneva: World Health Organization; 2018.
32. Office of Diversion Control. Drug Disposal Information. Available at https://www.deadiversion.usdoj.gov/drug_disposal/drug-disposal.html. Last accessed October 16, 2024.
33. Berger AM, O'Neill JF (eds). Principles & Practice of Palliative Care & Supportive Oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2022.
34. Doyle D, Woodruff R. The IAHPC Manual of Palliative Care. 3rd ed. Houston, TX: International Association for Hospice and Palliative Care Press; 2013.
35. Pergolizzi JV Jr, Mercadante S, Echaburu AV, et al. The role of transdermal buprenorphine in the treatment of cancer pain: an expert panel consensus. Curr Med Res Opin. 2009;25(6):1517-1528.
36. Scottish Partnership for Palliative Care. Scottish Palliative Care Guidelines. Available at https://www.palliativecareguidelines.scot.nhs.uk. Last accessed October 16, 2024.
37. U.S. Food and Drug Administration. Drugs@FDA: FDA-Approved Drugs. Available at https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Last accessed October 16, 2024.
39. Argoff CE, Silvershein DI. A comparison of long- and short-acting opioids for the treatment of chronic noncancer pain: tailoring therapy to meet patient needs. Mayo Clin Proc. 2009;84(7):602-612.
40. Lauretti GR, Oliveira GM, Pereira NL. Comparison of sustained-release morphine with sustained-release oxycodone in advanced cancer patients. Br J Cancer. 2003;89:2027-2030.
41. LexiDrug. Available at https://online.lexi.com. Last accessed October 16, 2024.
43. Shaiova L, Berger A, Blinderman CD, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165-176.
44. Nicholson AB, Watson GR, Derry S, Wiffen PJ. Methadone for cancer pain. Cochrane Database Syst Rev. 2017;(2):CD003971.
45. Dart RC, Woody GE, Kleber HD. Prescribing methadone as an analgesic [letter]. Ann Intern Med. 2005;143:620.
46. Drugs.com FDA Approves Nucynta ER (Tapentadol) Extended-Release Oral Tablets for the Management of Neuropathic Pain Associated with Diabetic Peripheral Neuropathy. Available at https://www.drugs.com/newdrugs/fda-approves-nucynta-er-tapentadol-extended-release-oral-management-neuropathic-pain-associated-3461.html. Last accessed October 16, 2024.
47. Vadivelu N, Timchenko A, Huang Y, Sinatra R. Tapentadol extended-release for treatment of chronic pain: a review. J Pain Res. 2011;4:211-218.
48. Schikowski A, Krings D, Schwenke K. Tapentadol prolonged release for severe chronic cancer-related pain: effectiveness, tolerability, and influence on quality of life of the patients. J Pain Res. 2014;8:1-8.
49. Blick SK, Wagstaff AJ. Fentanyl buccal tablet: in breakthrough pain in opioid-tolerant patients with cancer. Drugs. 2006;66(18):2387-2395.
52. Fallon MT, Laird BJA. A systematic review of combination step III opioid therapy in cancer pain: an EPCRC opioid guideline project. Palliat Med. 2011;25(5):597-603.
53. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454.
54. Dolinsky C, Metz JM. Palliative radiation therapy in oncology. Anesthesiol Clin North Am. 2006;24:113-128.
55. Fine PG. Palliative radiation therapy in end-of-life care: evidence-based utilization. Am J Hospice Palliat Med. 2002;19(3):166-170.
56. Lutz S, Spence C, Chow E, et al. Survey on use of palliative radiotherapy in hospice care. J Clin Oncol. 2004;22:3581-3586.
57. McCloskey S, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137.
58. Jarosek SL, Virnig BA, Feldman R. Palliative radiotherapy in Medicare-certified freestanding hospices. J Pain Symptom Manage. 2009;37(5):780-787.
59. Berrang T, Samant R. Palliative radiotherapy knowledge among community family physicians and nurses. J Cancer Educ. 2008;23(3):156-160.
60. Tilden VP, Drach LL, Tolle SW. Complementary and alternative therapy use at end-of-life in community settings. J Altern Complement Med. 2004;10(5):811-817.
61. Demmer C. A survey of complementary therapy services provided by hospices. J Palliat Med. 2004;7(4):510-516.
62. Kozak LE, Kayes L, McCarty R, et al. Use of complementary and alternative medicine (CAM) by Washington State hospices. Am J Hosp Palliat Care. 2008;25(6):463-468.
63. Dain AS, Bradley EH, Hurzeler R, Aldridge MD. Massage, Music, and Art Therapy in Hospice: Results of a National Survey. J Pain Symptom Manage. 2015;49(6):1035-1041.
64. Olotu BS, Brown CM, Lawson KA, Barner JC. Complementary and alternative medicine utilization in Texas hospices: prevalence, importance, and challenges. Am J Hosp Palliat Care. 2014;31(3):254-259.
65. Polubinski JP, West L. Implementation of a massage therapy program in the home hospice setting. J Pain Symptom Manage. 2005;30:104-106.
66. Choi TY, Lee MS, Kim TH, Zaslawski C, Ernst E. Acupuncture for the treatment of cancer pain: a systematic review of randomised clinical trials. Support Care Cancer. 2012;20(6):1147-1158.
67. Raub JA. Psychophysiologic effects of hatha yoga on musculoskeletal and cardiopulmonary function: a literature review. J Altern Complement Med. 2002;8:797-812.
68. Cepeda MS, Carr DB, Lau J, Alvarez H. WITHDRAWN: Music for pain relief. Cochrane Database Syst Rev. 2013;(10):CD004843.
69. Reynolds MW, Nabors L, Quinlan A. The effectiveness of art therapy: does it work? J Am Art Ther Assoc. 2000;17:207-213.
70. Schmidt C. Experts worry about chilling effect of federal regulations on treating pain. J Natl Cancer Inst. 2005;97(8):554-555.
71. American Medical Association. Ethics: Withholding or Withdrawing Life-Sustaining Treatment: Code of Medical Ethics Opinion 5.3. Available at https://code-medical-ethics.ama-assn.org/ethics-opinions/withholding-or-withdrawing-life-sustaining-treatment. Last accessed October 16, 2024.
72. American Medical Association. Ethics: Caring for Patients at the End of Life. Available at https://www.ama-assn.org/delivering-care/ethics/caring-patients-end-life. Last accessed October 16, 2024.
73. Bagdasarian N. A prescription for mental distress: the principles of psychosomatic medicine with the physical manifestation requirement in NIED cases. Am J Law Med. 2000;26:401-438.
74. Congress.Gov. S.3397 – Secure and Responsible Drug Disposal Act of 2010. Available at https://www.congress.gov/bill/111th-congress/senate-bill/3397. Last accessed October 16, 2024.
1. McCusker M, Jolkvosky M, Ruff R, et al. Palliative Care for Adults. 6th ed. Bloomington, MN: Institute for Clinical Systems Improvement; 2020. Available at https://www.icsi.org/wp-content/uploads/2021/11/PalliativeCare_6th-Ed_2020_v2.pdf. Last accessed October 19, 2024.
Mention of commercial products does not indicate endorsement.