The utility of aesthetic ultrasound as an adjunct modality available to clinicians to understand and elucidate various technical-, anatomic-, and treatment-related issues has gained increased relevance due to large number of aesthetic procedures performed by healthcare professionals across various medical disciplines. Modern aesthetic ultrasound units offer exceptional portability and convenience which can be used in daily clinic, office settings, and spas. More importantly, these technologies enable detailed and precision visualization that can safeguard potential complications of aesthetic procedures.
This course is designed to for clinicians and healthcare professionals currently performing aesthetic medicine.
This course is designed to provide clinicians and healthcare professionals currently performing aesthetic medicine with a practical concise guide and overview to using aesthetic ultrasound as a safe diagnostic aid to minimally invasive aesthetic procedures and as a point-of-care modality for patients who have undergone these procedures.
Upon completion of this course, you should be able to:
- Review the terminology and mechanics of diagnostic ultrasound technologies.
- Define the important biologic effects of ultrasound for clinicians.
- Review ultrasound techniques for pre-procedure planning, with understand basic tissue properties.
- Compare and contrast the ultrasound appearance of common cosmetic fillers.
- Discuss potential pitfalls to avoid when using ultrasound in aesthetic treatments.
- Identify residual unwanted cosmetic filler and safe methods of dissolution.
- Discuss ultrasound screening for RF-microneedling for nonsurgical blepharoplasty and evaluation of post-surgical blepharoplasty eye bag swelling.
- Review use of ultrasound for treatment of vascular occlusion complications of cosmetic fillers.
- Discuss key anatomic landmarks using ultrasound guidance.
Vincenzo Giuliano, MD, DABR, ARMDMS, is a licensed diagnostic radiologist in the State of Florida with a private consulting practice and former medical faculty member, with numerous peer-reviewed scientific publications in diagnostic radiology. He is unique as a practicing radiologist with specialized certification the American Board of Radiology and American Registry of Medical Diagnostic Medical Sonography, in addition to extensive clinical experience in esthetic injections and procedures in a private practice setting.
Contributing faculty, Vincenzo Giuliano, MD, DABR, ARMDMS, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John V. Jurica, MD, MPH
Mary Franks, MSN, APRN, FNP-C
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#90400: Ultrasound Applications in Aesthetic Practice
Diagnostic ultrasound is well established as a point-of-care modality in clinical decision-making, particularly with regard to intervention across various medical and surgical disciplines. While diagnostic ultrasound has remained a staple in medical practice for decades, application-specific use of this modality for aesthetic practice is limited. This course outlines potential uses and techniques of diagnostic ultrasound technology for routine aesthetic practice, while also providing relevant details regarding technology selection, basic points regarding operation, safety considerations, and basic image interpretation.
Cosmetic fillers, a staple of aesthetic practice, are amenable to targeted aesthetic ultrasound. Cosmetic fillers are soft tissue filler injections widely acknowledged as safe and straightforward treatment for aesthetic purposes of volume restoration and rejuvenation. These fillers are derived from various materials, most common including hyaluronic acid, calcium hydroxyapatite, and poly-L-lactic acid. The U.S. Food and Drug Administration (FDA) has approved more than 30 dermal fillers. According to the American Society for Aesthetic Plastic Surgery, an estimated 3,410,730 soft tissue filler injections were performed in the United States in 2020, ranking it as the second most common minimally invasive cosmetic procedure [4]. However, the growing popularity of these procedures has resulted in an expected proportional increase in associated adverse events. Inadvertent filler placement is becoming a more frequent occurrence. Other complications, once considered rare, are now recognized with increasing frequency, most serious being local tissue necrosis and arterial embolism [5,6]. Recent research and development of newer filler products have circumvented these concerns with enhanced efficacy and minimized complications through novel pharmaceutical design and techniques of administration but nevertheless remain highly user-dependent based on the experience of the injector [7].
The dominance of aesthetic medicine in recent years, and resulting new variables and potential risks, has created the need for an effective and versatile technology to address these concerns. This course expands on the use of a new discipline of aesthetic ultrasound as a valuable tool for aesthetic practice, with many novel potential applications useful even to the experienced clinician. When properly implemented, aesthetic ultrasound techniques can be applied to any ultrasound system, irrespective of ultrasound vendor.
This activity also provides a salient summary of the types of commercially available ultrasound units (including detailed discussion of piezoelectric versus microchip transducer systems) that can be used in aesthetics practice. Aesthetic ultrasound units are designed for limited or targeted use, not for total body application, typically consisting of a single handheld device (the imaging transducer) in combination with a base system or software. Some clinicians refer to the imaging transducer as imaging probe, a term now generally considered archaic.
Nearly all commercially available ultrasound systems operate on piezoelectric crystal technology for operation [8,9]. Pioneering research and development with the tech sector has produced newer engineering designs utilizing a vibrating membrane assembly with silicon microprocessor chip, replacing the conventional piezoelectric crystal systems [9,10]. Aesthetic ultrasound has unique technical requirements of higher spatial resolution of up to 20 MHz at the expense of a larger device footprint, presenting some physical limitations in scanning curved structures, such as facial convexity. Another limitation of aesthetic ultrasound is the lack of certain Doppler vascular applications, mainly a power Doppler (a higher-level application) function only available on larger, more expensive ultrasound systems equipped with suitable technology to visualize smaller lower flow vessels.
Most commercially available aesthetic ultrasound systems are equipped with conventional color and duplex Doppler capability that can be used to identify high-flow arteries amenable to imaging, such as in the case of facial aesthetic procedures, the superficial temporal artery (typically mapped prior to performing polydioxanone/PDO thread lifts) and the facial artery. The long course of the facial artery is routinely visible from its emergence from the mandible then oblique laterally to the lip commissure, terminating into the nasal ala; furthermore, it can be seen in profile(termed en face).
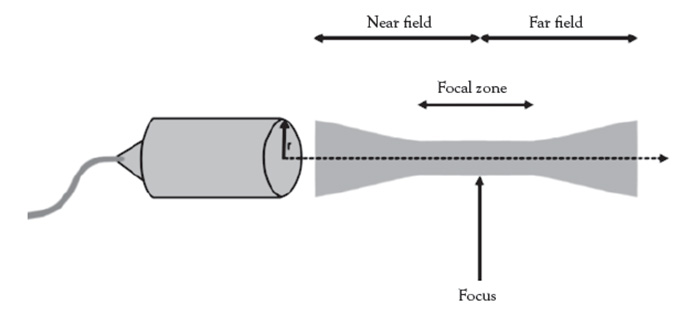
An important technique/application introduced in this course (not commonly used by aesthetic practitioners currently performing ultrasound) is the use of an acoustic standoff pad, which permits greater visibility of superficial soft tissues and vessels by placing the anatomy closer to the optimal focal zone of imaging, thereby optimizing near-field visibility [11,12]. Without this application, the anatomy of interest is placed too far superficially and greatly limits visualization by crowding superficial soft tissues within a thin space. The acoustic standoff pad is somewhat limited by the large lateral footprint of the ultrasound imaging transducer; therefore, it is better suited for use in combination with transducers of narrow width. The standoff pad technique is particularly advantageous in the visualization of superficial cosmetic fillers and mapping of imaging-guided dissolution treatments with hyaluronidase enzyme, when needed.
Finally, this course also discusses the use of aesthetic ultrasound technique as a useful application in the evaluation of lower lid eye bag evaluations, particularly in screenings for periorbital edema versus bulging fat and assessment of the integrity of the orbicularis oculi retaining ligament.
These applications are particularly advantageous during the planning stage. For instance, aesthetic ultrasound can be used to triage non-invasive methods (such as RF-microneedling) versus surgical blepharoplasty, recognizing that not all cases are amenable to treatment and should be referred for surgical blepharoplasty.
Both aesthetic medicine and radiologic imaging use terminology that are for a highly specialized professional audience. Aesthetic medicine is a combination of art and science involving procedures and techniques to improve and enhance the morphologic appearance, texture, and contour of skin, face, and body. This course references facial aesthetic techniques and technologies that are non-invasive or minimally invasive and be performed without or with local anesthesia.
The lexicon of minimally invasive procedures in aesthetic medicine denotes dermal or subcutaneous injections, punctures, or small incisions that do not require surgery. Therapeutic goals are not standardized and are generally subjective. Most methods and techniques are designed to minimize aging related to skin laxity, rhytids, and contour abnormalities. Desirable therapeutic goals include volumizing, rejuvenation, restoration, resurfacing, and correction, not specific to one standard or facial feature, and therefore variable from individual to individual. This course will not attempt to modify terminology used in the published literature. Several excellent resources are available to familiarize this audience. For the purposes of this course, the terms aesthetic medicine and cosmetic medicine referenced in the literature are interchangeable [1,2,3].
Similarly, diagnostic ultrasound uses a complex lexicon of terminology. For this course, the terms ultrasound and sonography commonly referenced in the literature can be regarded as interchangeable. Comprehensive discussion of diagnostic ultrasound as a discipline is beyond the scope of this course. However, new users of this technology are encouraged to refer to available literature, which reviews basic understanding and use of ultrasound-specific language/terminology. This course will not attempt to modify terminology currently used in the published literature. The term aesthetic ultrasound implies use of the same technology used in routine clinical diagnostic ultrasound; however, it is limited in scope and confined to specific anatomic references, procedures, and methods applicable to aesthetic medical practice.
Ultrasound is a type of mechanical (not electrical) energy collectively known as sound energy. The normal human range of sound is between16 Hz 20,000 Hz (1 MHz = 1 million cycles per second), with higher end of the spectrum seen in children and young adults. Ultrasonic frequencies beyond 20,000 Hz and can be applied in both therapeutic and diagnostic applications. The frequencies generally used in therapy are typically between 1–3 MHz for deeper tissues and 2–20 MHz in diagnostic ultrasound studies. Ultrasound beams are emitted by handheld devices, called transducers, which are mechanized high-frequency sound generators [13,14]. Figure 1 illustrates a schematic of a basic ultrasound system.
The mechanics of the ultrasound beam is complex. A simplified explanation is a sound wave of longitudinal energy that causes compression and rarefaction, meaning that it will cause oscillation of a fixed point of body tissue, rather than motion of that tissue within the wave itself [15]. Although ultrasound presents a theoretical concern for heat generation and tissue destruction by cavitation, the frequencies used in both therapeutic and diagnostic ultrasound are non-thermal in nature [16]. The energy absorption to body tissues is minimal, but different depending on depth and type of body tissue. In addition, the ultrasound energy is pulsed rather than continuous to further mitigate energy absorption by body tissues [17]. Another important property of ultrasound energy source is that it is non-ionizing, and therefore produces no teratogenic risks in pregnancy and developing fetuses [18].
All body tissues will impede the passage of ultrasound waves, with variability based on tissue density and elasticity. The presence of air and metal will effectively impede nearly 100% of the beam resulting in no effective transmission. For this reason, a coupling gel (typically water-based) is used to maximize contact between the imaging transducer and body tissues for optimal ultrasound wave transmission [19].
Nearly all commercial ultrasound systems are based on piezoelectric (quartz crystal) transducer technology, in which the crystal produces ultrasound waves when an electric current is passed through it. The ultrasound transducer is fabricated from a single piece of piezoelectric quartz material engineered into individual (generally less than 1,000 elements, called an array) attached to shielded electric cables,in three basic transducer configurations: linear, curvilinear, and sector/phased array [8,9]. The main advantages of piezoelectric systems is superior image quality and ease of use [22,23]. Disadvantages include limited application of the transducer to single body part, durability, higher cost, inherently lower frame rate (slower image acquisition), and susceptibility to damage when dropped [24,25].
Newer commercially available non-piezoelectric technologies use a novel engineering of silicon microchip transducers, which can produce targeted ultrasound sound waves for virtually anybody imaging application using simulated linear, curved, and phased-array capabilities,with additional platforms for artificial intelligence tools [26,27]. This pioneering new engineering is still in development and has not replaced conventional piezoelectric systems.
Butterfly was the original portable hand-held silicon microchip unit. Engineering consists of 8,960membrane (MEMS) elements on a single transducer-on-CMOS microchip, each with individual control and processing circuitry for sending and receiving ultrasound signals through the acoustic lens of a handheld transducer [28]. The MEMS membrane assembly produces an ultrasound sound wave when stimulated by electrical current [29]. Disadvantages include some reduction of image quality related to smaller footprints, limiting lateral resolution [30]. Advantages include lower cost, durability, higher frame rates (sharp images, faster processing), long battery life, total body applications, and military-grade durability, sustaining drops up to 4 feet and forces up to 100 G [10].
Mindray is a state-of-the-art proprietary system on chip (SOC) technology in a compact laptop style workstation, providing ultra-high resolution imaging capability of 20 MHz, and incredibly long battery life of eight hours [32]. Principle differences between Butterfly and Mindray units is that Butterfly is a single probe with multiple array configurations (for multiple body applications), while Mindray is limited to attachable single array probes (not regarded as a limitation for aesthetic application when using a single transducer setup).
This section summarizes basic parameter setting considerations intended as a general guide that can be applied to any ultrasound system, irrespective as to whether a fixed or portable ultrasound system. For facial aesthetics, a tranducers generally consist of linear transducer configuration designed for visualization of superficial structures. These imaging tranducers will typically have a higher frequency of 7–20 MHz [33]. Low frequency transducers below 7 MHz are not suitable for aesthetic ultrasound and should be avoided [34]. Higher frequency transducers are not designed to penetrate deep tissue depths of greater than 6 cm; deeper structures or thicker tissues generally require a curved array transducer in a lower frequency range in the range of 3 MHz,designed for increased depth penetration [35,36].
Detailed diagnostic ultrasound user settings are beyond the scope of this course. However, rudimentary knowledge of a few important basic user settings can be sufficient for routine aesthetic application. These include depth, gain, and Doppler settings.
Depth is a modifiable setting set by the user, on the imaging parameter screen. As a general rule, set the depth to 1 cm deeper than the intended target to place the anatomy of interest in the center of the focal zone, where the detail will be seen with the highest spatial resolution [37].
Gain is a modifiable setting set by the user, on the imaging parameter screen. The gain function allows the user to darken or brighten the image to optimize visualization of tissues at various depths [38].
Color Doppler is a modifiable more advanced setting that is beyond the scope of most basic aesthetic practices. However, when mastered, it a very useful tool in assessing variations in anatomical course of larger arteries, such as branches of the facial artery near the nasal ala, corners of the lip and mouth, and angle of the mandible. The rectangular color Doppler window should be centered over the artery of interest [39]. Color Doppler is most suitable for visualization of the superficial temporal and facial arteries, which are easily detectable high-flow vessels.
Ultrasound does not produce ionizing radiation, as in the case of x-ray or computed tomography scans. However, ultrasound does produce acoustic energy that does interact with human tissues, and is therefore considered relevant [40,41]. Bioeffects, both thermal and non-thermal in nature, need to be considered. Commercially available diagnostic ultrasound units are designed for safe application; no latent harmful biologic effects have been shown in humans [42]. However, scans should be performed setting the amount of acoustic exposure as low as reasonably achievable (known as the ALARA principle) [43].These biological effects are reported by the manufacturer as two main FDA-required standard preset values that appear on the image display as thermal index and mechanical index.
Thermal index (TI) is an indicator of the potential temperature increase resulting from the friction of tissues interfaces caused by acoustic energy [44]. Mechanical index (MI) is an indicator of non-thermal effects of cavitation from rarefaction of the ultrasound beam [45].
Standard good practices should include making sure factory default settings are integrated into the diagnostic ultrasound image display and performing periodic monitoring of the mechanical index and thermal index values. Minimize scan times to obtain required diagnostic information in the shortest time possible. Whenever possible, keep the transducer moving except when recording diagnostic images. Also apply special care to minimizing acoustic exposure to sensitive tissues (such as the eyes, most applicable when doing facial aesthetic ultrasounds) [46,47].
In pre-procedure planning, the use of an ultrasound gel standoff pad is optimal. The device allows optimized detection and specificity of superficial lesions and peripheral vessels that may be otherwise too superficial to visualize. The device places the anatomy of interest deeper into the focal zone (Figure 2), optimizing visualization of the lesion of interest. The technique is particularly useful when using lower resolution ultrasound systems. Currently available aesthetic ultrasound units do not provide power Doppler feature needed to visualize ultra-fine vascular structures, a technology only available on conventional larger complex, expensive state-of-the-art ultrasound imaging systems used in medical clinics and hospital settings.
Ultrasound images involve of spectrum of echogenicity, a medical term for the tissue property of the ability of the sound wave to reflect or transmit in surrounding tissues, each with a visible difference in contrast (from dark to bright) on the imaging screen [48].
Fluid, as can be seen with cystic lesions, are completely devoid of signal (decreased echogenicity) and appears uniformly black (termed anechoic) due to decreased acoustic impedance of the ultrasound wave [49].
Soft tissue encompasses a gray-scale spectrum of possible acoustic properties (heterogeneous echogenicity), ranging from tendons and ligaments on the lower end of acoustic impedance, and appear darker (hypoechoic) to muscle and dermal layers. Subcutaneous fat has the highest acoustic impedance and appears the brightest (hyperechoic) [36].
Calcium and air, with almost complete ultrasound wave impedance, results in a shadowing and scattering of sound waves (devoid of echogenicity), as can be commonly seen with bone and hollow, air-containing structures [51].
Cosmetic filler shows a variable appearance (from anechoic to hypoechoic), depending on composition and age [52].
Cosmetic fillers have continued to take center stage as safe and effective restoration of facial volume, recontouring, and additional collagen biostimulatory effect, leading to improved skin quality, as validated by their long-term safety, stability, and efficacy. The most commonly used temporary fillers are conjugated hyaluronic acid (HA) fillers and calcium hydroxylapatite (CaHa) [53]. Permanent injectable fillers, such as polymethylmethacrylate (PMMA), are less desirable because they do not break down (termed biodegradable) [54]. HA filler is a preferred agent because it is similar to naturally occurring HA in the body, which forms an integral part of the extracellular matrix for mechanical structure of various body tissues, including skin, muscle, and bone [55].
The FDA designates fillers based on the biodegradability of the filler. Main criteria include absorbable, temporary, semi-permanent, and non-absorbable or permanent [56]. Examples of absorbable/temporary fillers are HA and CaHa; semi-permanent fillers are poly-L-lactic acid (PLLA); and an example of non-absorbable/permanent fillers is PMMA [57]. Criteria for biodegradability are based on how these agents break down. Temporary fillers absorb within 18 months. Temporary fillers use FDA-approved biodegradable materials absorbed by the body overtime [58]. Permanent fillers do not absorb within 24 months. For practical purposes, permanent fillers persist indefinitely in tissue [59]. Semi-permanent fillers, such as PLLA combine absorbable material for immediate effect and carrier until a nonabsorbable material induces fibroblast stimulatory effects (in more than 18 months)[60].
Cosmetic fillers can induce minimal tissue response (termed volumizers) or strong tissue reaction (termed stimulators) [61]. The most commonly used cosmetic fillers demonstrate considerable variability with regard to soft tissue contrast dependent on chemical composition and ability to integrate within normal tissues.
HA is the most commonly used filler in clinical practice. HA is a glycosaminoglycan disaccharide and natural constituent of the dermal extracellular matrix, cartilage, and connective tissue. HA is highly hydrophilic, enabling dermal hyaluronic acid to hydrate and cushion the skin and fill empty spaces within the extracellular matrix [62]. Dermal HA content declines with skin aging, which reduces water-binding capacity and elasticity, inducing volume loss and eventual development of rhytids and other aging features [63].
Unmodified HA readily dissolves in water to form a viscous gel. When injected into the dermis, unmodified HA is quickly degraded by natural tissue hyaluronidase and free radicals in the skin [64]. Most FDA-approved HA fillers are commercially prepared from non-animal stabilized HA synthesized fromStreptococcibacteria [65].
Liquid stabilized HA, similar to native hyaluronic acid, is rapidly degraded in vivo. To increase stability and longevity, manufacturers use crosslinking agents to bind HA polymer chains to each other, resulting in a gel resistant to enzymatic and free radical breakdown. Most fillers use 1,4-butanediol diglycidyl ether (BDDE) as a cross-linker. The HA acid manufacturing modification process, proprietary to each manufacturer, facilitates a wide spectrum of HA properties with variable gel hardness, lifting ability, tissue bio-integration properties that direct affect long-term gel viability. HA can bind 1,000 times its volume in water. This water-binding and space-filling effect underlies the principal volumizing efficacy of hyaluronic acid fillers [55,67].
The highly hydrophilic nature of commercial HA gels provides an ideal acoustic appearance on ultrasound. HA acts like fluid, when properly hydrated, appearing anechoic to hypoechoic, with good acoustic transmission. HA does have a variable appearance dependent on filler viscosity, age, and degree of tissue biointegration [68]. Freshly injected HA may not have a significant acoustic footprint and can be imperceptible to normal tissue; it is only when hydrated/fluid-bound and hydrophilic that it appears anechoic to hypoechoic, particularly with highly viscous HA preparations [69]. Acoustic enhancement posteriorly is a property of HA and hydrophilic preparations. An example of supraperiosteal placement of hyaluronic acid filler is illustrated inFigure 3.
CaHA is a mineral commonly found in human teeth and bones that functions as a scaffold for collagen ingrowth. CaHA filler contains CaHA microspheres as biodegradable particles suspended in an aqueous carboxymethylcellulose gel carrier. Once injected, the carrier gel gradually resorbs; the microspheres stimulate a fibroblastic response resulting in active physiologic remodeling of the extracellular matrix and long-term collagen deposition around the implant that promotes volumizing [70,71]. The microspheres eventually degrade into calcium and phosphate ions and are then excreted. Filler duration is around 18 months [72]. Radiesse (formerly Radiance) is the only FDA-approved CaHA filler [73]. This viscoelastic filler is ideal for supraperiosteal and deep fat placement, clinically indicated for correcting moderate-to-severe soft-tissue defects, facial folds, and rhytids, including nasolabial folds [74].
Calcium provides a characteristic acoustic signature on ultrasound. In contradistinction to HA filler, CaHA shows typical shadowing and reverberation artifact from calcium composition.
PLLA (Sculptra) is a biodegradable, biocompatible, synthetic polymer,historically used as resorbable sutures, orthopedic plates, urologic stents, and, later, for cosmetic use [75]. PLLA induces a subclinical inflammatory response that stimulates fibroblast proliferation, neocollagenesis, and type I collagen formation, leading to a progressive increase in dermal volume. PLLA provides global volume restoration in lean patients lacking sufficient dermal volume and thickness for space occupying fillers, an alternative to deep volumizing hyaluronic acid fillers that could be too expensive and difficult to use as suitable dermal scaffolding [76]. PLAA injection is recommended for the supraperiosteal injection in the temples, lateral brow, zygomatic area, maxillary area, mandibular area, mental area, and also in the subcutaneous fat in the mid-cheek regions and preauricular area [77].
PLLA presents with mixed or heterogeneous echogenicity, sometimes with acoustic shadowing. For practical purposes, the PLLA ultrasound appearance cannot be reliably distinguished from other cosmetic fillers.
The most important landmarks for aesthetic imaging involve the facial and submental regions, which supply the complex vascular structures and neurovascular bundles. These structures must be avoided in facial aesthetic injections commonly used in clinical practice, such as the reduction of facial wrinkles and contouring. Injectables include botulinum toxin, cosmetic fillers (such as hyaluronic acid and hydroxyapatite) [78].
Botulinum toxin temporarily blocks the neuromuscular junction of underlying muscles, thereby causing relaxation and effectively eliminating wrinkles [79].
Cosmetic fillers are used primarily for volume augmentation and contouring of facial concavities and hollows caused by atrophy and age-related collagen loss. Accidental injury to neurovascular bundles from inadvertent or misplaced injection can result in adverse clinical outcomes, such as vascular occlusion leading to tissue necrosis, and temporary or permanent sensory deficits [80,81].
More serious complications include rare peripheral nerve transection, with permanent anesthesia due to sensory nerve deficit, and compressive ischemic effects resulting in vasospasm with subsequent embolic phenomenon, blindness, and cerebral infarction [82,83].
Neurovascular bundles typically consist of nerves, arteries, and veins [84]. In an ideal clinical setting, an ultra-high frequency ultrasound transducer of 20 MHz equipped with power Doppler would provide the best possible visualization of the these structures (only available in large complex state-of-the-art ultrasound systems) [85]. However currently available aesthetic ultrasound units designed for aesthetic practice are generally less than 20 MHz and are not equipped with power Doppler capability to detect these very small vessels.
Another practical point is that standard total body ultrasound units equipped with 15–20 MHz transducers and power Doppler have relatively larger footprints, making them unsuitable for highly contoured facial structures. Phillips Lumify, GE V-Scan Air, and Butterfly iQ all use small footprints suitable for facial aesthetics. Clarius and Mindray provide the highest possible resolution (with Clarius having a larger footprint and heavier transducer weight, which could be limiting factors).
The preferred protocol involves the use of smallest possible footprint transducer (for simple ergonomic reasons) in combination with a gel standoff pad, which partially mitigates the problem of decreased near field resolution, particularly with color Doppler vessel signal.
Anecdotal experience has suggested that HA fillers have a much longer lifespan than initially assumed. Prospective MRI studies have shown that HA fillers designed to dissolve within 6 to 12 months, surprisingly, can persist for up to 10 years post injection [86]. To date, there are no detailed clinical studies documenting a timeline of how long the dissolution process occurs in vivo, in human subjects [87]. Researchers have proposed a mechanism that the majority of dissolution occurs in the first initial weeks post-injection following a brief inflammatory response where hyaluronidase in produced by leukocytes causing a partial reduction in HA filler volume, a process that is dependent on the vascularity of the tissue. Once this inflammatory process has dissipated, the endogenous production of naturally occurring hyaluronidase is minimal [88]. Another consideration is a comparison between naturally occurring HA produced in the body, in vivo, compared to commercially prepared HA. Typical half-life of naturally occurring HA is one day or less in skin and dermis, compared with three weeks or less in cartilage. Commercial preparations of HA are typically cross-linked, making them more resistant to chemical breakdown, often lasting for months and years [89].
Ultrasound is a useful diagnostic tool in determining volume of residual "old" filler present and can assist in planning of volume and placement of "new" filler. Specific issues to consider with regard to undesirable "old" filler, is inappropriate filler depth placement, such as superficial placement resulting in Tyndall effect. Tyndall effect is used to describe the bluish hue or discoloration that is visible under the skin from superficial placement of HA filler [90]. Another consideration is inadvertent filler location placement, for example, above the annular retaining ligament, resulting in an exaggerated eye bag or more generalized eye bag swelling. Another consideration is the unintended migration of properly placed cosmetic filler, even when appropriate injection technique was utilized, such as in the case of properly injected supraperiosteal injection of HA filler below the malar fat pad in the cheek. The supraperiosteal malar injection of cheek filler can be problematic, particularly in patients with underdeveloped cheek bones (also referred anatomically as the zygomatic or malar bone). In cases of unwanted HA filler, precision guided ultrasound techniques could be used to administer hyaluronidase in order to dissolve unwanted filler[91,92].
Of the two commonly used temporary cosmetic fillers available commercially, HA filler is the most widely recognized and used [93]. Hydrated HA filler has an anechoic to hypoechoic appearance (similar to simple fluid), and stands out as distinctly darker than surrounding tissues, such as skin, dermis, and muscle. Some heterogeneity within the filler could be encountered, depending on age and maturity of the filler, probably the result of biointegration into surrounding tissues [94].
In clinical experience, the use of hyaluronidase can prove highly effective. Hyaluronidase are enzymes (endoglycosidases) can depolymerize HA leading to degradation by hydrolyzing the disaccharide linkages. Hyaluronidase should only be administered by a board-certified medical professional, and only FDA-approved brands should be used, such as Hydase, Hylenex, Amphadase, and Vitrase [95]. Under no circumstance should hyaluronidase be used from an unverified source. Literature references suggest 5 IU of hyaluronidase for each 0.1 mL of HA [96,97].
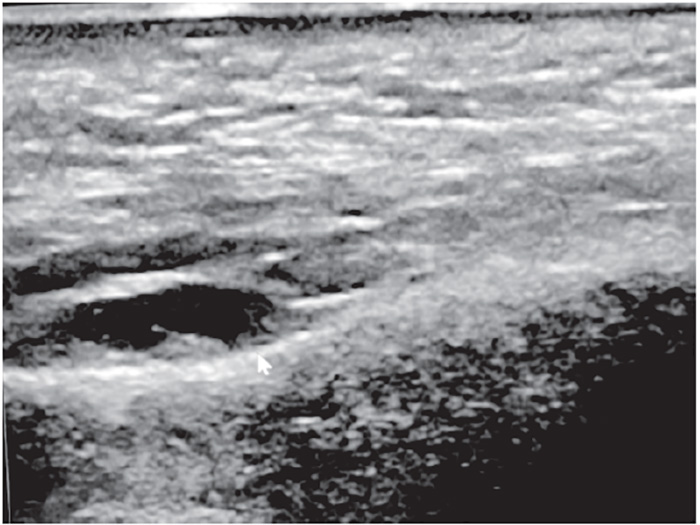
Typical ultrasound appearance of unwanted or residual HA filler is anechoic to hypoechoic, suggesting that the filler is reasonably well hydrated. One protocol consists of targeted treatment using microinjections of 15 IU, not exceeding 150 IU in total, administered in a radial pattern within unwanted (anechoic or hypoechoic) hydrated filler, dependent on volume of residual HA filler. Large quantities of HA filler (greater than 3 mL) could require repeat injections at one- or two-week intervals. Use of high-dose dose hyaluronidase flooding protocols (greater than 150 IU in a single session) is discouraged because of potential leakage into surrounding body tissues, risking an extended area of potential tissue breakdown. The effects of treatment are generally immediate, and a follow-up targeted ultrasound of the area shows no residual Ha filler (Figure 4) [98].
Ultrasound is also suitable for more problematic cases. Integrated or scarred HA filler placements show a less dark streak-like appearance on ultrasound (more on the isoechoic tissue spectrum), indicating a probable more fibrous rather than hydrated appearance. In clinical experience, these are generally resistant to hyaluronidase treatment and would require other methods of treatment (such as RF-microneedling).
In contradistinction, CaHa filler is composed of calcium-matrix and shows prominent acoustic shadowing (similar to bone), which is typical of a material with characteristically poor acoustic impedance [99]. CaHa is exceedingly difficult to dissolve chemically [100]. A preclinical pilot study using sodium thiosulfate showed statistical decreases in volume (attributed to dispersion), but with additional risks of tissue necrosis and hemorrhage; the study was terminated early due to side effects [101]. There are currently no long-term prospective studies justifying future continued use of sodium thiosulfate.
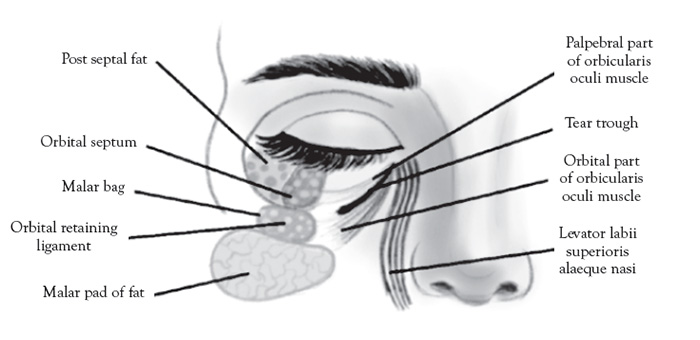
The orbicularis retaining ligament forms the superior border of the nasolabial and medial fat compartments. These two compartments combine to form the malar fat pad, an important structure in midface aging [102]. Elevation and repositioning of the malar fat pad is the primary target for midface aesthetic enhancement [103].
Blepharoplasty is a procedure to rejuvenate the upper and lower eyelids, either by surgical or non-surgical methods, and represents the fourth most common cosmetic surgical procedure in the United States [104]. RF-micro needling (such as AGNES technique) offers a viable non-surgical alternative for achieving long-term youthful and natural appearance to the eye and periorbital area [105]. Volume preservation and enhancement, rather than excessive tissue removal, define the modern blepharoplasty methodology [106]. The goal in the upper lid is to decrease eyelid laxity and increase visibility of the pretarsal space by micro-contouring [107]. The goal in the lower lid is to create a smooth lower lid surface and seamless transition into the cheek,combining micro-contouring with fat removal of the eye bag, if required [108].
The contents of the eye bag (Figure 5) are an important part of the screening protocol for non-surgical versus surgical blepharoplasty planning in which ultrasound can promising diagnostic utility. The contents of the eye bag (principally the distinction between fluid edema from fat) are equally important to elucidate during the screening procedure. The distinction of periorbital edema and fluid from normal fat can be tricky clinically, but easily elucidated with ultrasound.
Another indication for ultrasound is to address excessive periorbital swelling related to over-correction/over-filling of the tear trough area under the eye using cosmetic filler. This is the result of incorrect placement versus migration of filler above the orbicularis retaining ligament or due to superficial filler placement under the skin [109]. The mechanism of this phenomenon is unclear and difficult to treat clinically [110]. The only solution is to dissolve the filler and then replace the filler using modified (superolateral) technique [111].The orbicularis retaining ligament forms the superior border of the nasolabial and medial fat compartments, which combine to form, collectively, the malar fat pad, a recognized structure affected by midface aging [102]. Cosmetic fillers attempt to rejuvenate the midface by elevating and repositioning the malar fat pad via supraperiosteal injection [103].
Three distinct ultrasound gray-scale appearances can be anticipated in ultrasound screening evaluations of the malar fat pad. Periorbital edema/fluid is anechoic (appears dark on the scans), with posterior wall acoustic enhancement due to low acoustic impedance of water [112]. HA is anechoic to hypoechoic (appears dark to a shade less darker than fluid), a property that is variable, with mixed acoustic impedance, based on the type/viscosity, age of the filler, and degree it has integrated into the surrounding soft tissues [113]. Fat is hyperechoic (appears bright on the scans), due to high acoustic impedance, and easily distinguished from both fluid and HA filler [114].
Periorbital edema is relatively uncommon in routine outpatient practice. However, a careful screening should be performed to exclude premalar and cheek swelling; in such cases, hypothyroidism should be excluded prior to aesthetic treatments [115].
Lower lid blepharoplasty is a special consideration amenable to diagnostic ultrasound evaluation. Two common surgical approaches are the transconjunctival and skin-muscle flap blepharoplasties. The main distinguishing feature between the two procedures is that the transconjuctival approach does not violate the orbicularis oculi muscle, relying on a skin-only incision, in contradistinction to the skin-muscle flap technique that tightens the orbicularis oculi muscle, resembling a SMAS facelift [108].
Irrespective of technique, there are no standardized outcome criteria for either procedure [108]. Postoperative complications after blepharoplasty include hematoma, asymmetry, lagophthalmos, lower lid malposition, scleral show, dry eyes, frank lower lid ectropion, lateral canthal webbing, and chemosis. The most devastating complication after blepharoplasty is blindness that can occur as a result of globe injury, retrobulbar hematoma, and/or fat grafting; these complications are exceeding rare in experienced hands [108].
While reported complications and reoperation rates in the literature are generally low, they probably do not reflect actual outcomes encountered in common practice, as they are often published in series of experienced surgeons [108]. The duration of postoperative recovery after blepharoplasty is under-reported and probably underestimated [108]. Of particular interest is a phenomenon called chemosis, a bulbar conjunctival swelling that can occur with varying severity,mainly in the setting of lower blepharoplasty, with a reported occurrence of less than 12% and as high as 34% (in a single study) [117,118]. Multiple etiologies have been proposed for the development of chemosis, including inflammation, exposure, and lymphatic disruption.
Anatomical studies of the periorbital lymphatic drainage suggest the presence of a deep lymphatic drainage system that drains the conjunctiva and passes deep to the preseptal orbicularis piercing the orbicularis retaining ligament laterally at its junction with lateral orbital thickening. Consequently, procedures involving deep lateral dissection can theoretically increase the incidence of chemosis [108].
Reported treatments, including frequent lubrication of the conjunctiva with wetting drops, topical antibiotic/steroid ointments, and vasoconstrictive agents, are generally effective for mild chemosis, which is benign and self-limited [108]. However, there are no effective treatment strategies for more serious cases [108].
Ultrasound evaluations of eye bag swelling following blepharoplasty can prove useful in establishing a diagnosis of chemosis. Such evaluations should be reserved for cases exceeding six months duration and cleared by the cosmetic plastic surgeon, for which no explanation or viable treatment is offered. In clinical experience, a key diagnostic feature is a thin anechoic/hypoechoic fluid collection (which can be subtle, only 2–3 mm deep) lateral to the mid-pupillary line at the level of the infraorbital fissure, typically in a supraperiosteal location and contained by the orbicularis retaining ligament. Disruption of the lymphatic drainage of the conjunctiva is a potential etiology. Accordingly, treatments should be directed at improving lymphatic drainage (such lymphatic Hydrafacial massage), sometimes combined with cryotherapy, and have found moderate improvement of previously refractory cases. Hydrafacial is patented facial treatment device designed for exfoliation, cleansing, extraction, and hydration to the face using a vortex swirling action, with an additional lymphatic massage setting [119].
Vascular occlusion is a legitimate concern given the complex array of facial arterial anastomoses, asymmetry, and anatomic variation. Adverse outcomes appear related to the large number of cosmetic filler injections (more than 3 million procedures performed annually in the United States alone), and can be encountered even with experienced injectors. Most common complications are tissue ulceration, skin necrosis, and, rarely, blindness [120]. The FDA has issued a safety warning since 2015 [120]. The incidence of cosmetic filler related skin necrosis is 1:100,000 (or 0.001%). About 50% of complications occur in the glabellar region due to poor collateral circulation. The most common symptom of vascular occlusion is livedo reticularis, a netlike pattern of reddish-blue discoloration (occurring in 100% of patients), and decreased sensation (in 50% of patients). The most common sites of filler-related skin necrosis are the nose (33%) and nasolabial folds (30%). The most common site of filler-related blindness is the glabella (50%of cases) [121,122,123].
One of the principal advantages of HA fillers is their complete reversibility with hyaluronidase, an enzyme consisting of endoglycosidases that cleave the glycosidic bonds, inducing rapid depolymerization [124].
Hyaluronidase is the first-line treatment of vascular occlusion [125]. Published treatment protocols range from high-dose flooding regimen with a mean dose of 500 IU for ischemic area of 3 x 3 cm, versus ultrasonically guided pulsing of 35–60 units into the area daily for three to eight treatment sessions until normal skin color returns [126]. High doses increase the risk of retinal toxicity with inadvertent intravascular injections of the enzyme in the periorbital region. In most cases complete recovery is generally encountered in less than 12hours, somewhat longer in chin necrosis from injury to the larger submental artery. The mean time for complete reversal of vascular occlusion is 72 hours,with a maximum of 7 days. Successfully treated patients encountered no delayed ulceration, crusting, or skin damage [127,128].Ultrasound guidance offers a distinct advantage of precision delivery of lower doses, with the benefit of increased safety.
Aesthetic ultrasound is particularly useful in arterial mapping to identify potential "danger zones"of vascular occlusion and embolic complications. A discussion of relevant ultrasound-guided anatomic details is organized by zones in the upper, mid, and lower face, adapted from the current literature [129,130].
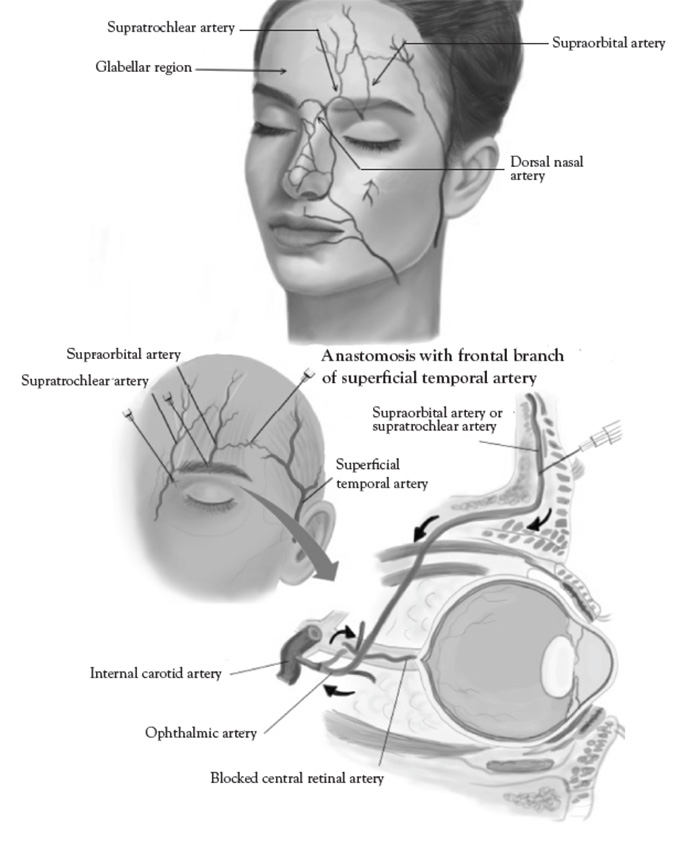
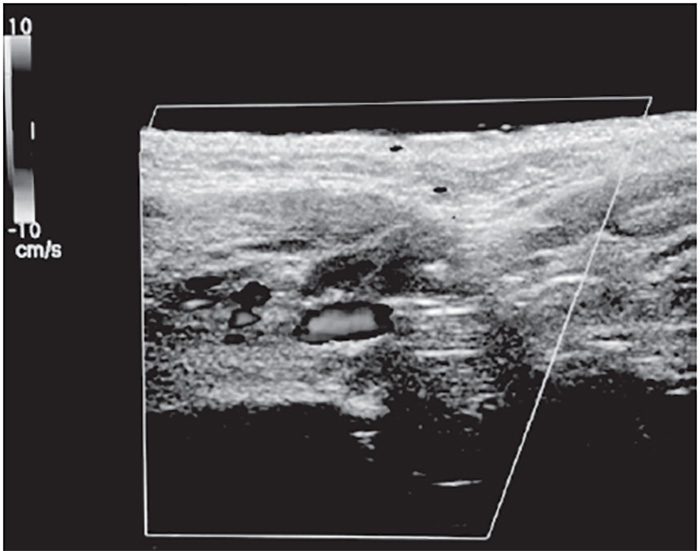
The superficial temporal artery is a branch of the external carotid artery and divides into frontal and parietal branches at the cranial portion of the zygomatic arch. The parietal branch is more medial and superficial to the temporal fascia, overlying the temporalis muscle, and must be avoided [131]. Inadvertent direct injection and/or compressive ischemia can cause retrograde embolization of the ophthalmic artery and blindness [132]. The superficial temporal artery must be carefully palpated and marked; cannula placement should be medial when placing polydioxanone (PDO) threads for midface lifts. The superficial temporal artery is visible with color Doppler ultrasound, when performed with proper technique (Figure 6).
The supraorbital groove and foramen form a subtle depression in the medial edge of the cranio-orbital margin of the frontal bone. The supraorbital groove and foramen contains the supraorbital artery (the terminal branch of the ophthalmic artery) and supraorbital nerve (first branch of the trigeminal nerve) [133]. The caliber of the supraorbital artery is too small and not amenable to conventional color Doppler ultrasound not otherwise equipped with power Doppler capability. However, the supraorbital groove can be localized on ultrasound and provides a precise location of the supraorbital artery in situations where it is not easily palpated.
Supraorbital(ophthalmic) nerve blockade is performed with a 27-gauge short needle and administering 2 cc 0.5% bupivacaine (or 50:50 mixture of lidocaine and bupivacaine) superficial to the periosteum upper/medial brow of the supraorbital notch,resulting in a characteristic sensory anesthesia dermatomal pattern [134].
The supratrochlear artery and branches are highly vascular and supplies the glabellar region. Inadvertent direct injection and/or compressive ischemia can cause retrograde embolization of the ophthalmic artery, blindness, and glabellar skin necrosis [135]. The glabella is the most common filler injection site leading to blindness [136]. Techniques for correcting deep rhytids in the glabellar region should include small intradermal microinjections and avoiding over filling [137]. The supratrochear artery is too small in caliber and not amenable to conventional color Doppler ultrasound not otherwise equipped with power Doppler capability.
The nasal and paranasal small network of arteries are too small in caliber and not amenable to conventional color Doppler ultrasound not otherwise equipped with power Doppler capability.
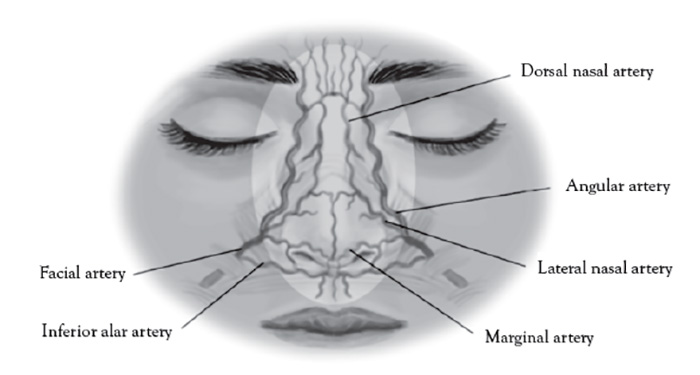
The dorsal nasal artery is a terminal branch of the ophthalmic artery and emerges from the medial orbital rim along the nasal septum [138]. The angular artery is the terminal portion of the facial artery and located at the lateral margin of the ala of the nose [138].
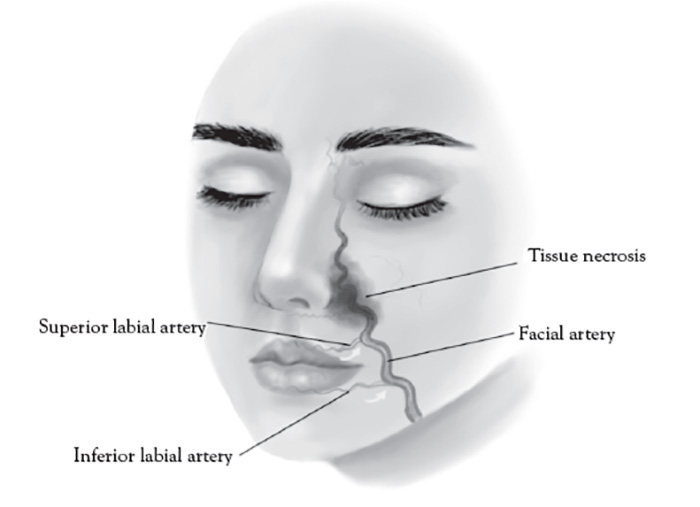
The paranasal area becomes a target in aesthetic practice, particularly with regard to injection of cosmetic fillers in achieving a "liquid rhinoplasty" or nasal contouring (Figure 7).Unlike other areas of cosmetic filler injections, midline nasal injections should be injected deep rather than superficial, because vascularity is more superficial, avoiding nasal bridge and ala [139]. Safe zones include midline middle third and lateral zone between the borders of the lateral nose and nasofacial groove. Avoid superficial injections of the alar tip and alar groove (avoid the crease) [139]. Nasal injection is the leading cause of tissue necrosis (Figure 8) [139,140].
The infraorbital groove and foramen contain the infraorbital artery (a terminal branch of the maxillary artery) and infraorbital nerve (a branch of the maxillary nerve, second branch of the trigeminal nerve) [141]. Approaches to tear trough injections of cosmetic filler in the cheek should be lateral to the mid-pupillary line, avoiding the infraorbital foramen, using lateral entry with preperiosteal needle track and deep retrograde injection technique [142]. The caliber of the supraorbital artery is too small and not amenable to conventional color Doppler ultrasound not otherwise equipped with power Doppler capability. However, the infraorbital groove can be localized on ultrasound and provides a precise location of the infraorbital artery in situations where it is not easily palpated.
Infraorbital nerve blockade (percutaneous approach) is performed with a 27-gauge short needle and administering 2cc 0.5% bupivacaine (or 50:50 mixture of lidocaine and bupivacaine)superficial to the periosteum medial mid-pupillary line just below the infraorbital ridge, resulting in a characteristic sensory anesthesia dermatomal pattern [143].
The path of the facial nerve originates at the brain stem, to temporal bone, via facial nerve canal, and exits the skull base via the stylomastoid foramen, superficially to the parotid gland, between the superficial and deep lobes at the level of the mandibular ramus [144]. Inadvertent injection of the parotid gland can cause temporary facial nerve paralysis (typically days to weeks) [145].
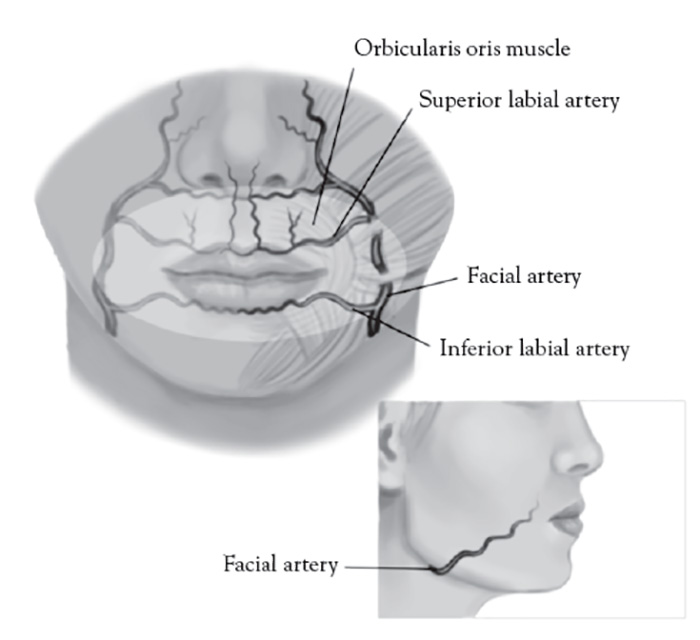
The facial artery is a branch of the external carotid artery, emerging superficial to the posterior belly of the digastric muscle, stylohyoid muscle, and submandibular gland, and coursing in a continuous oblique plane (1.5 cm)from the lateral commissure of the mouth to nasal ala along the lateral border of the nasolabial fold [146]. Proper injection technique for injecting cosmetic filler to the nasolabial fold is medial and very superficial, targeting the lower two-thirds intradermal plane, avoiding the nasal ala, where facial artery is most superficial [147].Inadvertent direct subcutaneous intravascular facial artery injection and/or compressive ischemia in the upper third of the nasolabial fold can result in an ocular embolism, blindness, and necrosis of the alar and malar region of the nose and cheek [148]. The facial artery is visible with color Doppler ultrasound when using proper technique (Figure 9).
The facial artery is located 15 mm lateral to the lip commissure and divides into the superior labial artery in the upper lip and inferior labial artery of the lower lip (Figure 10). These arteries are typically deep and run between the orbicularis oris muscle and oral mucosa [149]. General rules when addressing this facial area are [20,149]:
Always inject superficially and low-pressure injection
Never inject deeper than 3 mm from the skin or vermillion border (avoiding muscle)
Stay within a thumb width of the lip commissure
Inject slowly using retrograde technique
In addition, one should avoid the interior of the Cupid's bow, the orphiltrum [20,149]. The labial arteries are too small in caliber and not amenable to conventional color Doppler ultrasound not otherwise equipped with power Doppler capability.
The mental foramen is lateral to the mental protuberance of the chin at about the level of the lateral commissure of the lower lip, and contains the mental nerve (branch of the mandibular nerve of the third branch of the trigeminal nerve). The mental nerve provides sensation to the chin and lower lip [21].
The submental artery branch of the facial artery is seen in the inferior border of the mandible within the retromandibular groove at the mandibular notch, superficial to the mylohyoid muscle, and oriented in the long axis of the body of the mandible [31]. Avoid direct injections in the area of the retromandibular groove when applying local anesthesia to cheek and jawline [50]. The submental artery is visible with color Doppler ultrasound (Figure 11).
The hypoglossal nerve is the 12th cranial nerve that innervates the tongue muscles and forms a long medial course to the submental artery and vein along the inner body of the mandible, anterior the posterior, from the ear to chin [66]. Avoid direct injections in the area of the retromandibular groove [116].
The utility of aesthetic ultrasound as an adjunct modality available to clinicians to understand and elucidate various technical, anatomic, and treatment-related issues has gained increased relevance due to large number of aesthetic procedures performed by healthcare professionals across various medical disciplines. Modern aesthetic ultrasound units offer exceptional portability and convenience that can be used in daily clinic, office settings, and spas. More importantly, these technologies enable detailed and precision visualization that can safeguard potential complications of aesthetic procedures.
1. Griffiths D, Mullock A. Cosmetic surgery: regulatory challenges in a global beauty market. Health Care Anal. 2018;26(3):220-234.
2. Arsiwala SZ. Trends forfacial injectable therapies in medical aesthetics. J Cutan Aesthet Surg. 2018;11(2):45-46.
3. Gonzalez-Ulloa M, Flores ES. Senility of the face: basic study to understand its causes and effects. Plast Reconstr Surg. 1965:36:239-246.
4. Liao ZF, Cong LY, Li FW, et al.The research trend of soft tissue filler injection from 2000 to 2022: a bibliometric and visualized analysis. Plast Reconstr Surg Glob Open. 2024;12(2):e5579.
5. Galadari H, Krompouzos G, Kassir M, et al. Complication of soft tissue fillers: prevention and management review. J Drugs Dermatol. 2020;19(9):829-832.
6. Lee W, Koh IS, Oh W, Yang EJ. Ocular complications of soft tissue filler injections: a review of literature. J Cosmet Dermatol. 2020;19(4):772-781.
7. Heydenrych I, Kapoor KM, De Boulle K, et al. A 10-point plan for avoiding hyaluronic acid dermalfiller-related complications during facial aesthetic procedures and algorithms for management. Clin Cosmet Investig Dermatol. 2018:11:603-611.
8. Lee W, Roh Y. Ultrasonic transducers for medical diagnostic imaging. Biomed Eng Lett. 2017;7(2):91-97.
10. Le MPT, Voigt L, Nathanson R, et al. Comparison of four handheld point-of-care ultrasound devices by expert users. Ultrasound J. 2022;14(1):27.
11. Corvino A, Sandomenico F, Corvino F, et al. Utility of a gel stand-off pad in the detection of Doppler signal on focal nodular lesionsof the skin. J Ultrasound. 2020;23(1):45-53.
12. Catalano O, WortsmanX. Dermatology ultrasound. Imaging technique, tips and tricks, high-resolution anatomy. Ultrasound Q.2020;36(4):321-327.
13. Hangiandreou NJ. AAPM/RSNA physics tutorial for residents. Topics in US: B-mode US: basic concepts and new technology. Radiographics. 2003;23(4):1019-1033.
14. Abu-Zidan FM, Hefny AF, Corr P. Clinical ultrasound physics. J Emerg Trauma Shock. 2011;4(4):501-503.
15. Quarato CMI, Lacedonia D, Salvemini M, etal. A review on biological effects of ultrasounds: key messages for clinicians. Diagnostics (Basel). 2023;13(5):855.
16. Fowlkes JB, Bioeffects Committee of the American Institute of Ultrasound in Medicine. American Institute of Ultrasound in Medicine consensus report on potential bioeffects of diagnostic ultrasound: executive summary. J Ultrasound Med. 2008;27(4):503-515.
17. Maeda K, Kurjak A. The safe use ofdiagnostic ultrasound in obstetrics and gynecology. J Ultrasound Obstet Gynecol. 2012;6(3):313-317.
18. Torloni MR, Vedmedovska N,Merialdi, M, et al. Safety of ultrasonography in pregnancy: WHO systematic review of the literature and meta-analysis. Ultrasound Obstet Gynecol. 2009;33(5):599-608.
19. Casarotto RA, Adamowski JC, Fallopa F, Bacanelli F. Coupling agents in therapeutic ultrasound: acoustic and thermal behavior. Arch Phys Med Rehabil. 2004;85(1):162-165.
20. Lee SH, Gil YC, Choi YJ, Tansatit T, Kim HJ, Hu KS. Topographic anatomy of the superior labial artery for dermal filler injection. Plast Reconstr Surg. 2015;135(2):445-450.
21. Hur MS, Hu KS, Cho JY, et al. Topography and location of the depressor anguli oris muscle with a reference to the mental foramen. Surg Radiol Anat. 2008;30(5):403-407.
22. Manbachi A, CobboldRSC. Development and application of piezoelectric materials for ultrasound generation and detection. Ultrasound. 2011;19(4):187-196.
23. Shung KK, Zippuro M. Ultrasonic transducers and arrays. IEEE Eng Med Biol Mag. 1996;15(6):20-30.
24. Jung J, Lee W, Kang W, Shin E, Ryu J, Choi H. Review ofpiezoelectric micromachined ultrasonic transducers and their applications. J Micromech Microeng. 2017;27(11):113001.
25. Brown LF. Design considerations for piezoelectric polymer ultrasound transducers. IEEE Trans Ultrason Ferroelectr Freq Control.2000;47(6):1377-1396.
26. Rothberg JM,Ralston TS, Rothberg AG, et al. Ultrasound-on-chip platform for medical imaging, analysis, and collective intelligence. PNAS Nexus. 2021;118(27):e2019339118.
27. QiuZ, Piyawattanamatha W. New endoscopic imaging technology based on MEMS sensors and actuators. Micromachines (Basel). 2017;8(7):210.
28. Liu JY, Xu J, Forsberg F, Liu JB.CMUT/CMOS-based butterfly iQ - a portable personal sonoscope. Advanced Ultrasound in Diagnosis and Therapy. 2019;3(3):115-118.
29. Nayak G, Bolla V, Balivada SK, Prabhudev P. Technological evolution of ultrasound devices: a review. Int J Health Tech Innovation. 2022;1(3):24-32.
30. Salimi N, Gonzalez-Fiol A, Yanez ND, et al. Ultrasound image quality comparison between a handheld ultrasoundtransducer and mid-range ultrasound machine. POCUS J. 2022;7(1):154-159.
31. Yang HM, Won SY, Kim HJ, Hu KS. Neurovascular structures of the mandibular angle and condyle: a comprehensive anatomical review. Surg Radiol Anat. 2015;37(9):1109-1118.
32. Sonko ML, Arnold TC, Kuznetsov IA. Machine learning inpoint of care ultrasound. POCUS J. 2022;7(Kidney):78-87.
33. Mortada H, Al Mazrou F, Alghareeb A, Al Enezi M, Alalawi S, Neel OF. Overview of the role of ultrasound imaging applications in plastic and reconstructive surgery: is ultrasound imaging the stethoscope of a plastic surgeon? A narrative review of the literature. Eur J Plast Surg. 2023;46:15-24.
34. 34. Bhatta AK, Keyal U, Liu Y. Application of high frequency ultrasound indermatology. Discov Med. 2018;26(145):237-242.
35. Levy J, Barrett DL, Harris N, Jeong JJ, Yang X, Chen SC. High-frequency ultrasound inclinical dermatology: a review. Ultrasound J. 2021;13(1):24.
36. Rallan D, Harland CC. Ultrasound in dermatology: basicprinciples and applications. Clin Exp Dermatol. 2003;28(6):632-638.
37. Gorny KR, Tradup DJ, Hangiandreou NJ. Implementation and validation of three automated methods for measuring ultrasound maximum depth of penetration: application to ultrasound quality control. Med Phys. 2005;32(8):2615-2628.
38. Steffel CN, Brown R, Korcarz CE, et al. Influence of ultrasound systemand gain on grayscale median values. J Ultrasound Med. 2019;38(2):307-319.
39. StewartSF. Effects of transducer, velocity, Doppler angle, and instrument settings on theaccuracy of color Doppler ultrasound. Ultrasound Med Biol. 2001;27(4):551-564.
41. Barnett SB. Biophysical aspects of diagnostic ultrasound. Ultrasound Med Biol. 2000:26(Suppl 1):S68-S70.
42. Abramowicz JS, Barnett SB, Duck FA, Edmonds PD, Hynynen KH, Ziskin MC. Fetal thermal effects of diagnostic ultrasound. J Ultrasound Med. 2008;27(4):541-559.
43. Miller DL, Abo A, Abramowicz JS, et al. Diagnostic ultrasound safety review for point-of-care ultrasound practitioners. J Ultrasound Med. 2020;39(6):1069-1084.
45. Church CC, Labuda C, Nightingale K. A theoretical study of inertial cavitation from acoustic radiation force impulse imaging and implications for the mechanical index. Ultrasound Med Biol. 2015;41(2):472-485.
46. Barnett SB, Ter Haar GR, Ziskin MC, Rott HD, Duck FA, Maeda K. International recommendations and guidelines forthe safe use of diagnostic ultrasound in medicine. Ultrasound Med Biol. 2000;26(3):355-366.
47. 47. Kleinerman R, Whang TB, Bard RL, Marmur ES. Ultrasound indermatology: principles and applications. J Am Acad Dermatol. 2012;67(3):478-487.
48. Tsukahara K, Takema Y, Moriwaki S, Fujimura T, Imokawa G. Dermal fluid translocation is an important determinant of the diurnal variation in human skin thickness. Br J Dermatol. 2001;145(4):590-596.
49. Almuhanna N, Wortsman X, Wohlmuth-Wieser I, Kinoshita-Ise M, Alhusayen R. Overview of ultrasound imaging applications in dermatology. J Cutan Med Surg. 2021;25(5):521-529.
50. Marcuzzo AV, Šuran-Brunelli AN, Dal Cin E, et al. Surgical anatomy of the marginal mandibular nerve: a systematic review and meta-analysis. Clin Anat. 2020;33(5):739-750.
51. 51. Bagatin E, de Vasconcelos Nasser Caetano L, Marques Soares JL. Ultrasound and dermatology: basic principles and main applications in dermatologic research. Expert Rev Dermatol. 2013;8(5):463-477.
52. Urdiales-Gálvez F, De Cabo-Francés FM, Bové I. Ultrasoundpatterns of different dermal filler materials used in aesthetics. J Cosmet Dermatol. 2021;20(5):1541-1548.
53. Casabona G, Nogueira Teixeira D. Microfocused ultrasound in combination with diluted calcium hydroxylapatite for improving skinlaxity and the appearance of lines in the neck and décolletage. J Cosmet Dermatol. 2018;17(1):66-72.
54. Wortsman X, Quezada N. Ultrasound morphology of polycaprolactone filler. J Ultrasound Med. 2017;36(12):2611-2615.
55. Beasley KL, Weiss MA, Weiss RA.Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009;25(2):86-94.
56. Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 Suppl):7S-14S.
57. Sánchez-Carpintero I, Candelas D, Ruiz-Rodríguez R. Dermal fillers: types, indications, andcomplications. Actas Dermosifiliogr. 2010;101(5):381-393.
58. Fagien S, Klein AW. A brief overview and history of temporary fillers: evolution, advantages, and limitations. Plast Reconstr Surg.2007;120(6 Suppl):8S-16S.
59. Mortada H, Al Saud N, Alaithan B, Alhumsi T. Complications followingpermanent filler injection: a prospective cohort study and protocol of management. Plast Reconstr Surg Glob Open. 2022;10(11):e4687.
60. Christen MO. Collagen stimulators in body applications: a review focused on poly-L-lactic acid (PLLA). Clin Cosmet Investig Dermatol. 2022;15:997-1019.
61. Haddad S,Galadari H, Patil A, Goldust M, Al Salam S, GuidaS. Evaluation of the biostimulatory effects and the level of neocollagenesis of dermal fillers: a review. Int J Dermatol. 2022;61(10):1284-1288.
62. Al-Halaseh LK, Al-Jawabri NA, Tarawneh SK, et al. A review of the cosmetic use and potentially therapeutic importance of hyaluronic acid. J Appl Pharm Sci. 2022;12(7):34-41.
63. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule inskin aging. Dermatoendocrinol. 2012;4(3):253-258.
64. Landau M, Fagien S. Science ofhyaluronic acid beyond filling: fibroblasts and their response to the extracellular matrix. Plast Reconstr Surg. 2015;136(5 Suppl):188S-195S.
65. Saranraj P, Noorani AA, Naidu MA. Hyaluronic acid production and its applications: a review. Int J Pharm Biol Arch. 2013;4(5):853-859.
66. Bademci G, Yaşargil MG. Microsurgical anatomy of the hypoglossal nerve. J Clin Neurosci. 2006;13(8):841-847.
67. DeLorenzi C, Weinberg M, Solish N, Swift A. The long-term efficacy and safety of a subcutaneously injected large‐particle stabilizedhyaluronic acid-based gel of nonanimal origin in esthetic facial contouring. Dermatol Surg. 2009:35 Suppl 1:313-321.
68. Micheels P, BesseS, Flynn TC, Sarazin D, Elbaz Y. Superficial dermal injection of hyaluronic acid soft tissue fillers: comparative ultrasoundstudy. Dermatol Surg. 2012;38(7 Pt 2):1162-1169.
69. Micheels P, Besse S, Sarazin D, et al. Ultrasound and histologic examination after subcutaneous injection of two volumizing hyaluronic acid fillers:a preliminary study. Plast Reconstr Surg Glob Open. 2017;5(2):e1222.
70. Van Loghem J, Yutskovskaya YA,Werschler WP. Calcium hydroxylapatite: over a decade of clinical experience. J Clin Aesthet Dermatol. 2015;8(1):38-49.
71. Lorenc ZP, Bass LM, Fitzgerald R, Goldberg DJ, Graivier MH. Physiochemical characteristics of calcium hydroxylapatite (CaHA). Aesthet Surg J. 2018;38(suppl_1):S8-S12.
72. Ridenour B, Kontis TC. Injectable calcium hydroxylapatite microspheres (Radiesse). Facial Plast Surg. 2009;25(2):100-105.
73. Alam M, Havey J, Pace N, Pongprutthipan M, Yoo S. Large-particle calcium hydroxylapatite injection for correction of facial wrinkles and depressions. J Am Acad Dermatol. 2011;65(1):92-96.
74. Bass LS, Smith S, Busso M, McClaren M. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and efficacy results. Aesthet Surg J. 2010;30(2):235-238.
75. 75. Eason C, Snyder A, Favre C, Schlesinger T. Sculptra® - history andhow it is best used today. Dermatological Reviews. 2023;4(3):115-120.
76. Lam SM, Azizzadeh B,Graivier M. Injectable poly-L-lactic acid (Sculptra): technical considerations in soft-tissue contouring. Plast Reconstr Surg. 2006;118(3 Suppl):55S-63S.
77. Vleggaar D, Bauer U. Facial enhancement andthe European experience with Sculptra (poly-l-lactic acid). J Drugs Dermatol.2004;3(5):542-547.
78. Cox SE, Adigun CG. Complications of injectable fillers and neurotoxins. Dermatol Ther. 2011;24(6):524-536.
79. Thakker MM, Rubin PA. Pharmacology and clinical applications of botulinum toxins A and B. Int Ophthalmol Clin. 2004;44(3):147-163.
80. Cavallini M,Trocchi G, Heydenrych I, De Boulle K, Hendrickx B, Pirayesh A. Complications of absorbable fillers. In: Pirayesh A, Bertossi D, Heydenrych I (eds). Aesthetic Facial Anatomy: Essentials for Injections. Boca Raton, FL: CRC Press; 2020: 54-69.
81. Mehta P, Kaplan JB, Zhang-Nunes S. Ischemic complications ofdermal fillers. Plast Aesthetic Res. 2022;9:57.
82. De Boulle K. Complications from hyaluronicacid fillers. In: Bertossi D, Nocini R, Pirayesh I (eds). Non-Surgical Rhinoplasty. Boca Raton, FL: CRC Press; 2023: 112-120.
83. Sito G, Manzoni V, Sommariva R. Vascular complications after facial filler injection: a literature review and meta-analysis. J Clin Aesthet Dermatol. 2019;12(6):E65-E72.
84. Cotofana S,Lachman N. Arteries of the face and their relevance forminimally invasive facial procedures: an anatomical review. Plast Reconstr Surg. 2019;143(2):416-426.
85. Vidal Cofré NF, Martinez VM, Cornejo IN. Facial Doppler ultrasound in minimally invasive procedures. Int J Otorhinolaryngol Head Neck Surg. 2023;9(7):577-585.
86. Tal S, Maresky HS, BryanT, et al. MRI in detecting facial cosmetic injectable fillers. Head Face Med. 2016;12(1):27.
87. Wollina U, GoldmanA. Spontaneous and induced degradation of dermal fillers: a review. J Cutan Aesthet Surg. 2023;4010-4103.
89. Herrmann JL, Hoffmann RK, Ward CE, Schulman JM, Grekin RC. Biochemistry, physiology, and tissue interactions of contemporary biodegradable injectable dermal fillers. Dermatol Surg. 2018;44 Suppl 1:S19-S31.
91. Jung H.Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47(4):297-300.
92. Rzany B, Becker‐Wegerich P, Bachmann F, Erdmann R, Wollina U. Hyaluronidase in the correction of hyaluronic acid-based fillers: a review and a recommendation for use. J Cosmet Dermatol. 2009;8(4):317-323.
93. Radu A, Quereshy FA. Cosmetic fillers. In: Towne BM, Mehra P (eds). Neurotoxins and Fillers in Facial Esthetic Surgery. Hoboken, NJ: John Wiley & Sons, Inc; 2019: 47-61.
94. Grippaudo FR, Mattei M. The utility of high-frequency ultrasound in dermal filler evaluation. Ann Plast Surg. 2011;67(5):469-473.
95. Casabona G, Marchese PB, Montes JR, Hornfeldt CS. Durability, behavior, and tolerability of 5 hyaluronidase products. Dermatol Surg. 2018;44 Suppl 1:S42-S50.
96. Alam M, Hughart R, Geisler A, et al. Effectiveness of low doses of hyaluronidase toremove hyaluronic acid filler nodules: a randomized clinical trial. JAMA Dermatol. 2018;154(7):765-772.
97. 97. Murray G, Convery C, Walker L, Davies E. Guideline forthe safe use of hyaluronidase in aesthetic medicine, including modified high-dose protocol. J Clin Aesthet Dermatol. 2021;14(8):E69-E75.
98. Sharma SC, Lahiri A. Use of hyaluronidase in plastic surgery: a review. J Plast Reconstr Aesthet Surg. 2021;74(7):1610-1614
99. Jiang L, Yuan L, Li Z, Su X, Hu J, Chai H. High-frequency ultrasound of facial filler materials in the nasolabial groove. Aesthet Plast Surg. 2022;46(6):2972-2978.
100. Robinson DM. In vitro analysis of the degradation of calcium hydroxylapatite dermal filler: a proof-of-concept study. Dermatol Surg. 2018;44 Suppl 1:S5-S9.
101. Merkel EA, Worley B, Christensen RE, et al. In vitro and ex vivo investigation of intralesional sodium thiosulfate as a reversal agent for calcium hydroxylapatite soft tissue filler. J Invest Dermatol. 2022;142(11):3125-3127.
102. Yousuf S, Tubbs RS, Wartmann CT, Kapos T, Cohen-Gadol AA. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32(5):427-436.
103. 103. Chia CY, Almeida MWR, Ritter PD, de Aquino Nery E. Malar fat pad repositioning in facelifting: a simple technique of suspension and fixation. Aesthet Surg J. 2010;30(6):790-797.
104. Gao H, Wu D, Jie X, et al. Global research trends and perspectives of blepharoplasty: a 20-year bibliometric analysis based on web of science. Aesthet Plast Surg. 2023;47(2):654-665.
105. Majidian M, Moy RL. Commentary on: a single-center trial to evaluate the efficacy and tolerability of four microneedling treatments on fine lines and wrinkles of facial and neck skin in subjects with Fitzpatrick skin types I-IV: an objective assessment using non-invasive devices and 0.33-mm microbiopsies. Aesthet Surg J. 2021;41(11):NP1619-NP1 620.
106. Miotti G, Zeppieri M, Pederzani G, Salati C, Parodi PC. Modern blepharoplasty: from benchto bedside. World J Clin Cases. 2023;11(8):1719-1729.
107. Rohrich RJ, Villanueva NL, Afrooz PN. Refinements in upper blepharoplasty: the five-step technique. Plast Reconstr Surg. 2018;141(5):1144-1146.
108. 108. Alghoul M. Blepharoplasty: anatomy, planning, techniques, and safety. Aesthet Surg J. 2019;39(1):10-28.
109. Anido J, Fernández JM, Genol I, Ribé N, Pérez Sevilla G. Recommendations for the treatment oftear trough deformity with cross-linked hyaluronic acid filler. J Cosmet Dermatol. 2021;20(1):6-17.
110. Hamed-Azzam S, Burkat C, Mukari A, et al. Filler migration to the orbit. Aesthet Surg J. 2021;41(6):NP559-NP566.
111. Shamban A, Clague MD, von Grote E, Nogueira A. A novel and more aesthetic injection pattern formalar cheek volume restoration. Aesthetic Plast Surg. 2018;42(1):197-200.
112. Schelke L, Liplavk O, Cotofana S, Shah-Desai S, Velthuis P. Periorbital venous stasis may be involved with filler induced malar edema - a duplex ultrasound-imaging-based case series. J Cosmet Dermatol.2023;22(12):3246-3251.
113. Schelke LW, Van Den Elzen HJ, Erkamp PPM, Neumann HAM. Use of ultrasound to provide overall information on facial fillers and surrounding tissue. Dermatol Surg. 2010;36 Suppl 3:1843-1851.
114. Schelke L, Velthuis PJ, Lowry N, et al. The mobility of the superficial and deep midfacial fat compartments: an ultrasound-based investigation. J Cosmet Dermatol. 2021;20(12):3849-3856.
115. Kim BJ, Kazim M. Prominent premalar and cheek swelling: a sign of thyroid-associated orbitopathy. Ophthalmic Plast Reconstr Surg. 2006;22(6):457-460.
116. Mingazova LR, Karpova EI, Murakov SV, et al. Iatrogenic glossopharyngeal neuropathy in aesthetic practice: a case report. Plast Reconstru Surg Glob Open. 2022;10(3):e4166.
117. Pacella SJ, Codner MA. Minor complications after blepharoplasty: dry eyes, chemosis, granulomas, ptosis, and scleral show. Plast Reconstr Surg. 2010;125(2):709-718.
118. Prischmann J, Sufyan A, Ting JY, Ruffin C, Perkins SW. Dry eye symptoms and chemosis following blepharoplasty: a 10-year retrospective review of 892 cases in a single-surgeon series. JAMA Facial Plast Surg. 2013;15(1):39-46.
119. Costeloe A, Newman J. Aesthetician role in facial plastic surgery and systemic therapy for healthy skin. Facial Plast Surg Clin North Am. 2023;31(4):557-566.
120. Rauso R, Sesenna E, Fragola R, Zerbinati N, Nicoletti GF, Tartaro G. Skinnecrosis and vision loss or impairment after facial filler injection. J Craniofac Surg. 2020;31(8):2289-2293.
121. 121. Beleznay K, Carruthers JDA, Humphrey S, Carruthers A, Jones D. Update on avoidingand treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39(6):662-674.
122. Kato JM, Matayoshi S. Visual loss after aesthetic facial filler injection: a literature review on an ophthalmologic issue. Arq Bras Oftalmol. 2022;85(3):309-319.
123. Hwang CJ,Chon BH, Perry JD. Blindness after filler injection: mechanism and treatment. Facial Plast Surg Clin North Am. 2021;29(2):359-367.
124. Khan N, Niazi ZR, Rehman FU, et al. Hyaluronidases: a therapeutic enzyme. Protein Pept Lett. 2018;25(7):663-676.
125. Kim DW, Yoon ES, Ji YH, Park SH, Lee BI, Dhong ES. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg. 2011;64(12):1590-1595.
126. King M, Convery C, Davies E. This month's guideline: the use of hyaluronidase in aesthetic practice (v2.4). J Clin Aesthet Dermatol. 2018;11(6):E61-E68.
127. Cohen BE, Bashey S, WysongA. The use of hyaluronidase in cosmetic dermatology: a review ofthe literature. J Clin Investigat Dermatol. 2015;3(2):7.
128. Bachmann F, Erdmann R, Hartmann V, Wiest L, Rzany B. The spectrum of adverse reactions after treatment with injectablefillers in the glabellar region: results from the Injectable Filler Safety Study. Dermatol Surg.2009;35 Suppl 2:1629-1634.
129. Sykes JM, Cotofana S, Trevidic P, et al. Upper face: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(5 Suppl):204S-218S.
130. Freytag DL, Frank K, Haidar R, et al. Facial safe zones for soft tissue filler injections: a practical guide. J Drugs Dermatol. 2019;18(9):896-902.
131. Koziej M, Trybus M, Hołda M, et al. The superficial temporal artery: anatomical map for facial reconstruction and aesthetic procedures. Aesthet Surg J. 2019;39(8):815-823.
132. Lee JG, Yang HM, Hu KS, et al. Frontal branch of the superficial temporal artery: anatomical study and clinical implications regarding injectable treatments. Surg Radiol Anat. 2015;37(1):61-68.
133. Kleintjes WG. Forehead anatomy: arterial variations and venous link of the midline forehead flap. J Plast Reconstr Aesthet Surg. 2007;60(6):593-606.
134. Kohan J, Edalatpour A, Seitz AJ, Cho DY, Gander BH. Nerve blocks utilized inthe face: a comprehensive review. FACE. 2024;5(1):182-191.
136. Carruthers JDA, Fagien S, Rohrich RJ, Weinkle S, Carruthers A. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134(6):1197-1201.
137. Sahan A, Karaosmanoglu N, Ozdemir Cetinkaya P. A new three-point filler technique tomaximize safety for the correction of glabellar rhytids: evaluation of 50 patients. J Cosmet Dermatol. 2020;19(6):1311-1315.
138. Choi DY, Bae JH, Youn KH, et al. Topography of the dorsal nasal artery and its clinical implications for augmentation of the dorsum of the nose. J Cosmet Dermatol. 2018;17(4):637-642.
140. Kassir R, Venkataram A, Malek A, Rao D. Non-surgical rhinoplasty: the ascending technique and a 14-year retrospective studyof 2130 cases. Aesthet Plast Surg. 2021;45(3):1154-1168.
141. Lee JH, Hong G. Definitions of groove and hollowness of the infraorbital region and clinical treatment using soft-tissue filler. Arch Plast Surg. 2018;45(3):214-221.
142. Trinh LN, Grond SE, Gupta A. Dermal fillers for tear trough rejuvenation: a systematic review. Facial Plast Surg. 2022;38(3):228-339.
143. Aggarwal A, Kaur H, Gupta T, et al. Anatomical study of the infraorbital foramen: abasis for successful infraorbital nerve block. Clin Anat. 2015;28(6):753-760.
144. AlghoulM, Codner MA. Retaining ligaments of the face: review ofanatomy and clinical applications. Aesthet Surg J. 2013;33(6):769-782.
145. McElwee TJ, Poche JN, SowderJC, Hetzler LT. Management of acute facial nerve and parotid injuries. Facial Plast Surg. 2021;37(4):490-499.
146. Koziej M, Trybus M, Hołda M, et al. Anatomical map of the facial artery for facial reconstruction and aesthetic procedures. Aesthet Surg J. 2019;39(11):1151-1162.
147. Lee HJ, Won SY, O J, et al. Thefacial artery: a comprehensive anatomical review. Clin Anat. 2018;31(1):99-108.
Mention of commercial products does not indicate endorsement.