Pancreatic cancer, also referred to as pancreatic ductal adenocarcinoma (PDAC), is the most lethal solid malignancy, predicted to become the second leading cause of cancer death in the United States by 2030. The complexity of this aggressive cancer has been vexing to investigators and tragic for patients and their families. It is now clear that even early-stage PDAC is a systemic disease and that new-onset metabolic and neuropsychiatric symptoms/syndromes are prodromal rather than comorbid or secondary. This recognition has also called for a re-thinking of pancreatic cancer from a more integrative, multi-system perspective.
- INTRODUCTION
- EPIDEMIOLOGY
- PATHOPHYSIOLOGY
- PANCREATIC CANCER SCREENING
- CLINICAL EVALUATION OF PANCREATIC CANCER
- THE DIAGNOSTIC AND STAGING WORKUP
- TREATMENT APPROACHES FOR PANCREATIC CANCER
- PALLIATION AND SYMPTOMATIC MANAGEMENT
- CONSIDERATIONS FOR NON-ENGLISH-PROFICIENT PATIENTS
- CONCLUSION
- Works Cited
- Evidence-Based Practice Recommendations Citations
This course is designed for physicians, nurses, and other members of the interprofessional healthcare team involved in the care of patients with pancreatic cancer.
The purpose of this course is to provide an updated clinical review of pancreatic cancer for healthcare professionals. It is intended to address knowledge gaps, enhance clinical skills, promote risk assessment and disease prevention, and guide appropriate management of patients with the disease.
Upon completion of this course, you should be able to:
- Outline the epidemiology of and risk factors for pancreatic cancer.
- Describe the pathophysiology of pancreatic cancers.
- Identify high-risk patients for diagnostic screening for pancreatic cancer.
- Describe key aspects of the clinical evaluation of patients with suspected pancreatic cancer.
- Select the appropriate tools for diagnosis and staging of pancreatic cancer.
- Apply models of assessing the functional performance status of patients with diagnosed pancreatic cancer.
- Discuss the role of resection in pancreatic cancer treatment, including most appropriate approaches.
- Compare and contrast chemotherapy regimens used in the treatment of pancreatic cancer.
- Describe the use of radiation therapy as a component of pancreatic cancer treatment according to evidence-based guidelines.
- Evaluate available interventions to manage symptoms and provide palliative care to patients with pancreatic cancer.
Mark Rose, BS, MA, LP, is a licensed psychologist in the State of Minnesota with a private consulting practice and a medical research analyst with a biomedical communications firm. Earlier healthcare technology assessment work led to medical device and pharmaceutical sector experience in new product development involving cancer ablative devices and pain therapeutics. Along with substantial experience in addiction research, Mr. Rose has contributed to the authorship of numerous papers on CNS, oncology, and other medical disorders. He is the lead author of papers published in peer-reviewed addiction, psychiatry, and pain medicine journals and has written books on prescription opioids and alcoholism published by the Hazelden Foundation. He also serves as an Expert Advisor and Expert Witness to law firms that represent disability claimants or criminal defendants on cases related to chronic pain, psychiatric/substance use disorders, and acute pharmacologic/toxicologic effects. Mr. Rose is on the Board of Directors of the Minneapolis-based International Institute of Anti-Aging Medicine and is a member of several professional organizations.
Contributing faculty, Mark Rose, BS, MA, LP, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John M. Leonard, MD
John V. Jurica, MD, MPH
Mary Franks, MSN, APRN, FNP-C
Randall L. Allen, PharmD
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#90241: Pancreatic Cancer
Pancreatic ductal adenocarcinoma (PDAC) is relatively uncommon but carries a dismal prognosis. An estimated 64,000 new cases of PDAC are diagnosed each year, and the number is increasing at the rate of 0.5% to 1.0% per year [18,134]. Although pancreatic cancer accounts for 3% of newly diagnosed cancers (by comparison, prostate, lung, breast, and colorectal account for 48%), it is the third leading cause of cancer deaths and is projected to become the second-leading cause of cancer-related deaths by 2030 [18]. Onset of disease is insidious, and there is no highly effective screening modality to enable early detection of PDAC. Most patients present with locally advanced (30% to 35%) or metastatic (50% to 55%) disease at diagnosis [134]. The overall five-year survival rate has improved somewhat over the past two decades, from 5% in 2005, to 13% in 2024 [1,18]. However, almost half (47%) of patients with pancreatic cancer have late-stage disease at diagnosis, with a five-year survival rate of 3% [18].
There are four fundamental challenges to early diagnosis and reduction in mortality from PDAC: pancreatic anatomy, rapid disease progression, systemic effects, and limited treatment options. For patients with resectable disease at initial evaluation, surgery followed by adjuvant chemotherapy is the standard therapeutic approach, with the anticipation of median overall survival of 54.4 months [134]. However, only 15% of patients present with localized tumor amenable to surgical resection. Moreover, two-thirds of annual new cases are adults older than 65 year of age, a subset lacking the resilience aggressive surgery and chemotherapy require to achieve optimal treatment outcomes.
The pancreas is situated deep within the retroperitoneal space of the upper abdomen, behind the stomach and between the aorta and its major upper abdominal branches. Shielded from detection, pancreatic tumors often grow around and encase these vessels, making the cancer inoperable in nearly 85% of patients [2]. With this aggressive cancer, more than 50% of patients have locally invasive and/or distant metastases at diagnosis, and micrometastases are already present in most patients undergoing resection for apparently localized tumors [2,3,4].
Up to 80% of patients presenting with PDAC have cachexia, a wasting syndrome induced by the physiologic effects of the cancer. Cachexia dramatically weakens patients, limiting their ability to withstand aggressive treatment. Poor treatment tolerance by patients with cachexia is evidenced by decreased survival after resection or chemotherapy [2].
The complex tumor microenvironment and heterogeneity of gene mutations make PDAC one of the most drug-resistant cancers. Standard treatment options have limited effectiveness, and disease progression is usually rapid, with low complete responses to chemotherapy and radiotherapy [1,4]. Multi-agent chemotherapy regimens have a survival benefit of two to six months over what is achieved with single-agent chemotherapy [18]. For the 5% to 7% of patients with metastatic PDAC expressing specific pathogenic germline variants, such as BRCA1/2, maintenance therapy with olaparib (a poly adenosine diphosphate-ribose polymerase inhibitor) is an option that improves progression-free survival following initial platinum-based therapy (134).
Surgical resection of the pancreas with microscopically free margins (R0 resection) followed by chemotherapy remains the only realistic option for complete remission, but this is potentially achievable in only a fraction of patients [4,5]. Nonetheless, incremental gains have been increasingly frequent over the past decade, and more substantive gains are anticipated, pending clinical trial results. This course will describe the current standard of care for patients with pancreatic cancer and present information that may help increase earlier detection of this malignancy and improve the symptom burden and quality of life in these patients, regardless of disease stage.
Clinical practice guidelines for patients with pancreatic cancer have been published by the American Society of Clinical Oncology (ASCO), the NCCN (National Comprehensive Cancer Network), the American Society for Radiation Oncology (ASTRO), the European Society for Medical Oncology (ESMO), the National Institute for Health and Care Excellence (NICE), and others [6,7,8,9,10,11,12,13,14,15]. The recommendations are largely concordant on what constitutes multidisciplinary standards of care in the management of pancreatic cancer [2,16].
Most pancreatic cancers arise in the exocrine pancreas (95%). Tumors of the endocrine pancreas (<5%) are distinct from exocrine pancreas cancers and will not be discussed in this course [4].
PDACs account for more than 95% of exocrine pancreatic cancers. Other subtypes include acinar carcinoma, pancreaticoblastoma, and neuroendocrine neoplasia. PDAC and pancreatic cancer are commonly used as interchangeable terms in the literature and will be in this course [17].
During 2024 in the United States, an estimated 64,440 people will be diagnosed with pancreatic cancer, which represents 3.3% of all new cancer cases and the 11th most common new cancer diagnosis. The median age at diagnosis is 70 years [18].
Approximately 1.7% of men and women will be diagnosed with pancreatic cancer at some point during their lifetime, based on 2017–2019 data. In 2021, an estimated 100,669 people were living with PDAC in the United States [19].
With an estimated 51,750 deaths in 2024, pancreatic cancer is the third leading cause of cancer death (after lung and colorectal cancer) in both men and women. As the incidence is increasing by 0.5% to 1.0% per year, it is expected to become the second leading cause of cancer death by 2030 [18,19,20].
Pancreatic cancer stage at diagnosis strongly influences the length of survival, as shown by data from 2014 to 2020 (Table 1) [19]. The five-year survival of PDAC, 12.8%, remains the lowest of all common cancers [19,21].
Pancreatic cancer is more common among men than women, and the incidence rate increases with age. During the period 2017–2021, persons 65 to 84 years of age (median age: 70 years) accounted for 57% of newly diagnosed cases [19]. The annual pancreatic cancer incidence and mortality rates (per 100,000 persons) for all races were higher among men (15.4 and 12.9) than women (12.0 and 9.8). The rates were highest for Black men (17.7 and 15.3), followed by non-Hispanic White men (16.0 and 13.2). Rates were lower for Hispanics and lowest for Asian/Pacific Islanders and American Indian/Alaska Natives [19].
Using statistical models for analysis, the National Cancer Institute finds that age-adjusted rates for new pancreatic cancer cases have been rising on average 0.9% each year over 2012–2021, while age-adjusted death rates have been rising on average 0.2% each year between 2013 and 2022 [19]. Underlying these trends is a combination of an aging population, a longer lifespan, and the high prevalence of obesity and diabetes [11,18]. In 2015, lost earnings from person-years of life lost from pancreatic cancer were estimated at more than $6 billion [2].
In examining PDAC survival disparities over 2004–2015, the unadjusted median overall survival was slightly longer for White patients than Black patients (6.6 months vs. 6.0 months). Decreased survival for Black patients persisted after controlling for sociodemographic parameters. Conversely, controlling specifically for clinical parameters (e.g., disease stage, treatment) found a modest survival advantage for Black patients [22].
Black patients with PDAC present at younger ages with more advanced disease than White patients, possibly suggesting differences in tumor biology. Black patients receive less treatment stage-for-stage and fewer surgeries for resectable PDAC than White patients; these findings may be only partly associated with socioeconomic differences. In one study, when disease stage and treatment were controlled for, Black patients had no decrease in survival compared to other races [22].
Health professionals' implicit biases shape behaviors, communications, and interactions, which then produce differences in diagnoses and ultimately treatments and interventions. Implicit biases are subtle and unconscious and may unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider.
Racial and socioeconomic differences in surgical intervention rates, treatment at high-volume hospitals/centers, and morbidity and mortality rates have been noted, with the largest disparities between Black (and to a slightly lesser extent Hispanic) and White Americans [23]. Several factors are implicated, but implicit biases and insurance status are identified as potentially modifiable contributors.
The most common recognized risk factor for pancreatic cancer is cigarette smoking followed by obesity. Others include pancreatitis, diabetes, and family history of pancreatic cancer (Table 2) [13,24]. Periodontal disease is increasingly linked to pancreatic and other gastric cancers. Chronic pancreatitis substantially elevates the risk of developing pancreatic cancer and represents an opportunity for surveillance and monitoring. Most importantly, new-onset hyperglycemia or diabetes is now recognized as an early symptom of PDAC in an otherwise asymptomatic patient. Many recognized risk factors are modifiable for prevention of pancreatic cancer.
Cigarette smokers have at least a two-fold greater risk for pancreatic cancer than nonsmokers. The risk increases directly with daily number of cigarettes consumed and duration of smoking. In heavy smokers with polymorphism in the carcinogen-metabolizing enzyme gene glutathione S-transferase theta 1 (GSTT1), the risk is up to five-fold greater [25,26].
Excess risk decreases with smoking cessation. The risk of pancreatic cancer among current smokers (relative risk: 2.5) decreased 48% two years after smoking cessation, and within 10 to 15 years after cessation, it approximated that of nonsmokers [26].
In the United States, estimates indicate that 11% to 32% of deaths from PDAC are attributable to tobacco smoking. It is estimated that cessation of smoking could eliminate up to 25% of pancreatic cancer deaths [24,26].
Limited evidence suggests alcohol consumption may be associated with risk of developing PDAC, but findings of population-based studies are inconsistent. In pooled cohort data of 1.5 million light, heavy, or never-drinkers, heavy drinkers had a greater relative risk of developing PDAC than never-drinkers (relative risk: 1.29) or light drinkers (relative risk: 1.36). Light drinkers had no difference compared to never-drinkers (relative risk: 0.96) [27].
Most studies have assumed additivity between average effects of smoking and alcohol and oversimplified their impact on burden of pancreatic cancer. However, the combined effect of smoking and total alcohol intake on risk of PDAC is likely non-additive. It appears that only heavy consumption of liquor (but not wine or beer) increases the risk of PDAC in ever-smokers [27].
Obesity (defined as a mass index [BMI] >30) during early adulthood is associated with a greater risk of certain cancers and earlier onset. Several studies have reported that the incidence of early-onset pancreatic cancer has been increasing in parallel with the expanding prevalence of obesity. Other obesity-related cancers increasing in incidence and early-onset include colorectal cancer, multiple myeloma, and cancers of the uterus, gallbladder, and kidney. An analysis of incidence data from 25 state cancer registries covering 14.7 million invasive cancers diagnosed between 1995 and 2014 found the incidence of PDAC was increasing among adults 25 to 49 years of age, with steeper rises in successively younger birth cohorts [135]. Diets high in processed meat, high-fructose beverages, and saturated fat were associated with obesity, diabetes, and PDAC. Obesity is associated with a 20% to 40% higher mortality rate from PDAC, and obesity at an older age is associated with lower overall survival [13; 28].
Although BMI is widely used as a marker for general adiposity, measures of visceral obesity show a stronger correlation with metabolic syndrome, insulin resistance, and certain gastrointestinal (GI) malignancies. The proximity to visceral organs and drainage via the portal system may explain the strong correlation of inflamed visceral adipose tissue (VAT) in obese subjects with metabolic dysfunction and pancreatic cancer [29].
There is some evidence that higher consumption of red/processed meat is associated with elevation in pancreatic cancer risk, but other studies have failed to identify dietary risk factors for PDAC [11]. Pancreatic cancer incidence may be lower in persons with higher intake of fresh fruits and vegetables rich in folate and lycopenes (e.g., tomatoes) [30].
A link between vitamin D and risk for pancreatic cancer is inconsistent, but some data suggest low plasma 25-hydroxyvitamin D levels may increase the risk for pancreatic cancer, especially in those with low retinol/vitamin A intake [31]. Coffee and tea consumption are not associated with pancreatic cancer risk, despite early reports to the contrary [24].
Numerous studies and meta-analyses have found systemic/nonmodifiable factors that increased the relative risk, hazard ratio, or odds ratio of developing pancreatic cancer. These include individuals with greater height (relative risk: 1.81); individuals with blood groups A, AB, and B (hazard ratio: 1.32, 1.51, and 1.72, respectively); and patients with hepatitis B infection (odds ratio: 1.50) or systemic lupus erythematosus (hazard ratio: 1.43). Biologic explanations for some of these associations are not yet understood, and some data may have potential confounders. Infectious etiologies warrant more investigation [11,32].
Periodontitis describes a chronic inflammatory response to a disease-associated, multispecies bacterial community in the subgingival region. Periodontal disease is associated with pancreatic cancer, even when controlling for gender, smoking, BMI, diabetes, and alcohol consumption [33]. The inflammatory processes of periodontitis occur locally, but systemic dissemination of inflammatory mediators, subgingival species, and bacterial components contribute to digestive cancers (including PDAC) by activating proinflammatory pathways, inducing gene expression related to cell proliferation, apoptosis, and immune responses linked to carcinogenesis, cell migration, invasion, and metastasis [34].
A high-risk subgroup for PDAC are patients with chronic pancreatitis, often secondary to chronic alcohol use disorder, smoking, hypertriglyceridemia, diabetes, or renal failure [2]. Patients with chronic pancreatitis show a 26-fold increase in risk of developing PDAC. This risk increases with duration. Among patients with chronic pancreatitis of 20 years' duration, approximately 5% will progress to PDAC.
Concomitant smoking enhances the risk of neoplastic progression [2,35]. Hereditary pancreatitis further increases the risk of pancreatic cancer by more than 50-fold. In these individuals, the cumulative risk of pancreatic cancer by age 70 years is 40% [24].
Pancreatic cancer has complex relationships with diabetes and obesity that are only recently becoming understood. A population cohort study underscored the complex relationship between metabolic abnormalities and PDAC. Glycemic status, insulin resistance, and hyperinsulinemia were independently associated with an increased risk of pancreatic cancer mortality, even in individuals without diabetes [36].
The association between pancreatic cancer and diabetes was noted as early as 1833, clearly documented by the 1930s, and characterized in a large cohort of patients with pancreatic cancer from Mayo Clinic in 1958 [37]. Several meta-analyses have greatly refined the risk-factor status of diabetes.
Long-standing (i.e., more than five years) diabetes (both type 1 and type 2) is associated with increased risk of developing PDAC [13]. The overall risk for PDAC increases 4- to 7-fold in those with diabetes of a duration less than three years [38]. The relative risk associated with diabetes levels off after five years, with a 1.5-fold greater risk [39]. Increased baseline hemoglobin A1C (HbA1C) levels correlate with subsequent development of PDAC [40].
Long-standing diabetes modestly increases the risk of PDAC, which decreases with diabetes duration [11,37]. The initial three-year period after diabetes diagnosis is high risk for PDAC, as confirmed by prospective pancreatographic screening [41].
With diabetes medications, insulin use has been associated with increased risk of PDAC, but this finding is attributed to reverse causality [11,42]. Metformin use in patients with diabetes and PDAC was associated with improved two-year survival (30.1% vs. 15.4%) and median overall survival (15.2 months vs. 11.1 months) in patients without metastases [43]. One metformin study reported negative findings [44].
Long-standing diabetes in patients who develop PDAC is associated with significantly lower overall survival (14.4 months vs. 21.7 months) and significantly higher mortality (harm ratio: 1.52) compared with patients without diabetes who develop PDAC [11,45].
Diabetes of the exocrine pancreas (formerly type 3c diabetes) is the second most common type of new-onset diabetes in adults (behind type 2 diabetes) [42]. Acute or chronic pancreatitis is one of the most prevalent risk factors for PDAC and the most frequent cause of diabetes of the exocrine pancreas. Pancreatitis leads to postpancreatitis diabetes mellitus in up to 83% of patients [42]. In a registry study involving 139,843 individuals, the proportion of pancreatic cancer was 3.1% among those with postpancreatitis diabetes mellitus, compared with 2.3% in those with type 2 diabetes followed by pancreatitis, 2.0% in those with pancreatitis alone, and 0.6% in individuals with type 2 diabetes alone [42].
Numerous clinical series have identified new-onset diabetes, weight loss, and soft tissue changes in patients with PDAC at diagnosis, but their inter-relationship and connection to PDAC remained unaddressed. From 2000 through 2015, temporal changes in the five years preceding PDAC diagnosis of 219 patients diagnosed with PDAC were compared to 657 controls [46]. From 60 to 30 months before PDAC diagnosis, patients did not significantly differ from controls. However, starting at 30 months prediagnosis, PDAC showed three distinct metabolic phases, each marked by onset and significant progressive worsening of one or more metabolic abnormalities [46]:
Phase 1, hyperglycemia (30 to 18 months before PDAC diagnosis): A significant proportion of patients develop hyperglycemia, without soft tissue changes.
Phase 2, pre-cachexia (18 to 6 months before PDAC diagnosis): Decreases in serum lipids, weight loss, and the first soft tissue change (subcutaneous abdominal tissue loss) are seen. A profile appears of advanced prediabetes (i.e., fasting blood glucose 120–126 mg/dL or A1c of 6% to 6.5%). In type 2 diabetes, this is associated with weight gain and hyperlipidemia due to insulin resistance. In PDAC, decreases in weight and serum lipids despite rising glucose levels are paradoxical.
Phase 3, cachexia (less than 6 months before PDAC diagnosis): Onset of muscle loss, visceral adipose tissue loss, and decreasing high-density lipoprotein. Continued decreases in all other serum lipids, subcutaneous abdominal tissue, and weight. Fasting blood glucose continues rising.
Based on evidence of increases in body temperature before PDAC diagnosis, browning and loss of subcutaneous abdominal tissue is estimated to begin 18 months before PDAC. Browning of white abdominal tissue is a mechanism of subcutaneous abdominal tissue loss in cancer; its purpose is to generate heat [46].
Symptoms of cachexia and muscle loss (e.g., anorexia, fatigue, reduced exercise tolerance) appear shortly (less than six months) before PDAC diagnosis. The onset of objective weight loss precedes PDAC diagnosis by one year or more. New-onset diabetes appears a median of six to nine months before PDAC diagnosis [46].
Cancer cachexia is a paraneoplastic syndrome characterized by pronounced weight loss and muscle wasting triggered by cancer-induced systemic inflammation [47]. Cachexia develops in about 80% of patients with PDAC during the disease course, often before the tumor is clinically apparent. Cachexia negatively impacts treatment response and survival, and one-third of patients with PDAC die from cachexia-associated complications, including impaired immunity and cardiopulmonary dysfunction. No curative treatments exist [47].
Pancreatic cancer-associated diabetes mellitus might be a major contributor to PDAC-induced cachexia. The co-occurrence is frequent, and the relationship between pancreatic cancer-associated diabetes and PDAC-induced cachexia was clarified in a 2020 study [47]. Compared with patients without pancreatic cancer-associated diabetes, those with pancreatic cancer-associated diabetes did not have a higher risk of cachexia, a greater degree of weight loss, or lower skeletal muscle mass. Among patients with cachexia, weight loss and skeletal muscle mass were comparable between patients with and without pancreatic cancer-associated diabetes. Fasting blood glucose levels and PDAC-derived diabetogenic factors did not correlate with weight loss or muscle mass or predict cachexia in patients with pancreatic cancer-associated diabetes. A notable finding was the consistently high prevalence of cachexia and muscle wasting regardless of tumor size and stage in PDAC [47]. These results argue against pancreatic cancer-associated diabetes and hyperglycemia in mediating PDAC-induced cachexia.
Cancer cachexia is characterized by systemic inflammation with resultant skeletal muscle breakdown and increased circulating amino acids to support tumor growth. Pancreatic cancer-associated diabetes is a metabolic strategy by PDAC to fuel tumor growth. PDAC cells have a high demand for glucose (termed "glucose addiction"); hyperglycemia promotes invasion and migration of PDAC cells. PDAC-induced cachexia and pancreatic cancer-associated diabetes are distinct metabolic reprogramming induced by PDAC cells to secure amino acids and glucose for tumor growth [47].
Unexplained weight loss/cachexia is a clue to occult PDAC, but a modality that can identify PDAC-induced cachexia is needed to take advantage of this screening opportunity [47]. Optimizing glycemic control may not alleviate weight loss or muscle wasting, and therapies targeting mediators of pancreatic cancer-associated diabetes may not protect against the development of cachexia [47]. Management of cachexia in patients with PDAC is discussed in detail later in this course.
PDAC is caused by somatic (acquired) and germline (inherited) mutations in specific cancer-associated genes. In PDAC, the accumulation of multiple combinations of gene mutations significantly perturbs major signaling pathways, leading to a malignant phenotype [13,48,49,50].
Like most solid tumors, PDACs are driven by mutations that disrupt intra- and extracellular networks that normally restrain abnormal growth, proliferation, survival, and invasion [51]. Four major genetic drivers are fundamental in nearly all PDACs. These involve mutational activation of the oncogene KRAS, and mutational inactivation of the tumor suppressor genes CDKN2A, TP53, and SMAD4[3,50,52,53]. Inactivation of genome maintenance genes that repair DNA damage is a third broad type of mutation in PDAC.
KRAS encodes a GTPase molecule that acts as a transducer for growth factor receptors on the cell surface. KRAS mutations dysregulate intrinsic GTPase activity, stimulating downstream pathways that drive uncontrolled cellular proliferation, angiogenesis, suppression of apoptosis, and evasion of immune response [54].
CDKN2A encodes the proteins p16 and p14ARF, which are both cell-cycle regulators. With loss of CDKN2A gene function, inactivation of p16 results in unchecked cell cycle progression and enhanced tumor cell proliferation [3,49]. TP53 encodes the protein p53, called the "guardian of the genome," which plays a central role in DNA repair, cell cycle arrest, and induction of apoptosis in response to DNA damage or cellular stress [55].
Inactivation of p53 (loss of function mutation) allows DNA damage to go unchecked with failed apoptosis and unregulated G1/S cell cycle transition. Mutant p53 can also gain pro-oncogenic activities (gain-of-function mutation), promoting cell proliferation, survival, angiogenesis, and metastases [54].
SMAD4 encodes the protein Smad4, a downstream effector of transforming growth factor-beta (TGF-b) signaling pathway. SMAD4 inactivation and loss of Smad4 promotes cancer progression by removing the early growth inhibitory effect of the TGF-b pathway and is associated with higher rates of distant metastasis and poorer prognosis [54].
Through pathways and somatic mutations that differ modestly in each lesion, PDAC develops from precancerous precursor lesions: pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasms (MCNs). The most common are PanINs (approximately 90%), and the least common are MCNs (<15%). However, all precursor lesions have key similarities [4,48,50]:
Early oncogene mutations initiate tumorigenesis.
Later loss of tumor suppressor genes drives tumor progression, high-grade dysplasia, and invasive cancer.
Increasing grades of dysplasia are associated with accumulation of somatic mutations in key driver genes.
PDAC develops in PanINs through a specific process [56]. First, mutational KRAS activation initiates pancreatic carcinogenesis. With tumor suppressor inactivation, cancer progresses. CDKN2A or SMAD4 are implicated in locally destructive disease; TP53 is involved in metastatic seeding; and concurrent SMAD4 and TP53 are often present in locally or metastatic dominant disease. IPMNs and MCNs often share the driver gene mutations and sequence of PanINs, but also show specific patterns.
Lesions involving the main pancreatic duct have a higher malignant potential than those in the branches, with the risk of malignancy at around 62% [11]. More than 90% of IPMNs are marked by activating mutations in the oncogene GNAS and/or inactivating mutations in the tumor suppressor gene RNF43[48,53,54]. GNAS mutation causes constitutive activation of adenylyl cyclase, with downstream effects driving proliferation. RNF43 encodes E3 ubiquitin-protein ligase, which functions as a tumor suppressor in the Wnt-signaling pathway. After the initiating oncogene mutation, the progression of IPMN resembles PanIN.
The PanIN Progression Model has been critical in shaping the perspective of how PDAC develops and progresses. PDAC arises through a specific sequence of genetic alterations over a gradual progression from early PanIN to late-stage metastatic disease [57,58,59].
The timeframe of PanIN progression has also been established. Based on computational modeling using autopsy cases, the estimated average time interval from initiation in normal cells to invasive ability (11.7 years), metastatic dissemination (6.8 years) and death (2.7 years) corresponds to an average of about 21 years from the initiating mutation until a patient's death [17].
Most cases with PDAC are diagnosed toward the end of this lifetime span, suggesting that poor prognosis is largely a function of late diagnosis in the natural history of PDAC, and that a golden opportunity of two or three years exists to diagnose "early" pancreatic cancer (i.e., Stage 0 or I) [60].
Chromothripsis, a recently identified phenomenon, is a catastrophic event causing tens to thousands of chromosomal rearrangements. Faced with hundreds of DNA breaks, the cell's DNA repair machinery attempts to rescue the genome, but the result bears little resemblance to its original structure [61,62]. This genomic disruption can drive the development of cancer through DNA copy number changes, including deletion of tumor suppressor genes and increased copy number (amplification) of oncogenes [61].
A 2016 study of more than 100 whole genomes from pancreatic cancer tumors found evidence of at least one chromothripsis event in 65% of tumors, and most copy-number changes seemed to occur after such catastrophic genetic events. With evidence of chromothripsis in some PDACs and nongradual tumorigenesis that defies the established mutational sequence, a punctuated equilibrium model was proposed, dividing tumor development into two major events [63]:
A cancer-initiating event: PDAC preneoplasms acquire extensive mutation burden but remain non-invasive over a prolonged preneoplastic phase.
A cataclysmic cancer-transforming event: Chromothripsis induces DNA copy number changes, creating genomic instability and generating invasive clones with rapid dissemination and colonization of distant sites. Why chromothripsis occurs in PDAC is not yet understood.
Rather than being uniformly aggressive, PDAC exhibits clinical (e.g., variable patient survival) and disease (e.g., variable chemotherapy sensitivity) heterogeneity [64,65]. The first whole-genome description of PDAC in 2008 prompted great effort to advance a patient-tailored precision medicine approach that could better address this heterogeneity. Genetic alterations and molecular subtypes in PDAC were characterized and published. PDAC was shown mutationally dominated by the four driver genes and homogeneous. In general, the findings importantly informed the biology and familial predisposition of PDAC.
However, by 2019 it was apparent that PDAC disease heterogeneity cannot be explained by genetic mutations alone, and non-genetic mechanisms, including epigenetics and the tumorigenic microenvironment, were the path forward [21,56,59,62,64,65,66,67].
Epigenetic Factors
Broadly speaking, epigenetic changes influence gene expression, without altering the DNA sequence, through modifications of DNA or chromatin structures [4]. In PDAC, these include DNA methylation and non-coding RNAs (ncRNAs).
Gene expression in PDAC can be silenced through non-mutational inactivation by aberrant promoter methylation, including the driver gene p16/CDKN2A[49]. Aberrant ncRNA expression plays a considerable role in initiation, proliferation, and chemo-resistance of PDAC. Oncogenic microRNA-21 promotes both cell proliferation and apoptosis and targets negative regulators of KRAS, which further enhances signaling by this oncogene [50,54].
Pancreatic Tumor Microenvironment
PDAC is comprised of cancer cells within dense fibrotic stroma consisting of extracellular matrix and non-neoplastic (e.g., fibroblastic, vascular, immune) cells [3]. Also described as PDAC fibrosis, the stroma makes up most of the tumor mass. This network of neoplastic tissue and stroma (the pancreatic tumor microenvironment) acts as physical barrier to drug penetration and has elements that impede host immune surveillance and antagonize host anticancer immune responsiveness, facilitating PDAC growth, survival, and treatment failure [21,51,68]. Current anti-cancer therapy includes novel approaches designed to enhance sensitivity of PDAC to the host immune system and reverse the immunosuppressive effects of the tumor microenvironment [134].
Pancreatic cancer progresses in tandem with extensive stromal deposition of extracellular matrix, recruitment and activation of cancer-associated fibroblasts, and high interstitial fluid pressures, which compresses blood vessels, causing localized hypoperfusion, reduced vascularity, and tissue hypoxia [21,69]. Extracellular matrix remodeling biomechanically induces intracellular signaling and tumor-stellate cell crosstalk. PDAC cells signal to stellate cells and recruit macrophages and immune suppressor cells. In turn, stellate cells secrete factors that promote PDAC cell proliferation, migration, and suppression of apoptosis [51]. Biochemical activation of signaling pathways regulates PDAC cell survival and promotes tumor growth and metastasis. Down-stream effects include immunosuppression, disease progression, epithelial-mesenchymal transition (a key step of the metastatic cascade) and invasive potential, and chemotherapy resistance [3,21,69].
Exosomes (a macromolecule involved in RNA degradation) released by PDAC cells accumulate in other tissues to create a premetastatic niche by activating stellate cells and inducing remodeling of the host extracellular matrix, which facilitates cancer cell invasion and growth [59,69].
In addition to the somatic mutations driving pancreatic tumorigenesis in all PDACs, specific pathogenic germline alterations impose a predisposition to PDAC in some patients [48]. In many of these germline mutations, the oncogenic mechanism involves inactivation of DNA damage repair genes [49].
There are two broad categories of inherited risk for PDAC [26,70,71]:
Genetic predisposition or hereditary pancreatic cancer: Germline mutations in PDAC susceptibility genes are present.
Familial pancreatic cancer: Familial clustering of PDAC (i.e., at least one pair of affected first-degree relatives) without known germline mutations
Most patients with PDAC have no identifiable genetic factor nor familial history; such cases can be classified as sporadic. The frequency of identifiable pathogenic germline mutations in persons with PDAC is about 10% [136]. The most common pathogenic alterations are in BRCA1, BRCA2, and ATM, which with the other more rarely identified genetic alterations have an aggregate frequency of 3.8% to 9.7% [136]. Approximately 3% to 7% of patients with PDAC harbor a BRCA1 or BRCA2 gene mutation. Identification of pathogenic gene mutations is important for therapeutic purposes and useful for screening and surveillance of at-risk family members.
Several genetic syndromes are associated with specific genetic alterations with an increased risk for pancreatic cancer (Table 3) [48,54]. Germline mutations in familial atypical multiple mole melanoma syndrome (CDKN2A) and Li-Fraumeni syndrome (TP53) are core gene drivers in sporadic PDAC. Peutz-Jeghers syndrome is caused by germline inactivation of STK11, a tumor suppressor gene. Somatic STK11 mutations are observed in approximately 4% of pancreatic cancers, suggesting STK11 inactivation plays a role in both sporadic and familial forms [49].
PANCREATIC CANCER SUSCEPTIBILITY SYNDROMES AND MUTATIONS
| Category | Specific Syndromes and Germline Mutations | |||
|---|---|---|---|---|
| Gastrointestinal tract cancers |
| |||
| Solid tumor cancers |
| |||
| Chronic pancreatitis-associated syndromes |
| |||
| Neurodegenerative disease | Ataxia-telangiectasia (ATM) |
An estimated 10% to 15% of all pancreatic cancers are attributable to genetic causes. Pancreatic cancer aggregates in some families; 5% to 10% of individuals with pancreatic cancer have a family history of the disease [26,70,72]. Familial pancreatic cancer represents 90% of all hereditary PDAC cases. The relative risk of PDAC increases with the number of affected first-degree relatives.
A specific gene defect responsible for familial pancreatic cancer has not been identified, but a rare autosomal-dominant gene may be responsible, putting 0.4% to 0.7% of the population at risk for developing PDAC [26,70,72]. Details about the relative and lifetime risks of PDAC, and the other prevalent cancers associated with specific germline mutations in cancer susceptibility syndromes and familial pancreatic cancer, are summarized in Table 4.
PANCREATIC CANCER RISK IN PREDISPOSITION AND INHERITED CANCER SYNDROMES
| Syndrome | Gene(s) | Risk of PDAC | Other Cancers | |
|---|---|---|---|---|
| Relative | Lifetime | |||
| General population | – | 1 | 0.5% | – |
| Hereditary breast/ovarian cancer | BRCA1 | 2 to 3 | 1.2% to 2% | Breast, ovarian, prostate |
| BRCA2 | 3.5 to 10 | 2% to 10% | ||
| PALB2 | 15 | 5% to 10% | ||
| Familial atypical multiple mole melanoma | CKDN2A | 13 to 36 | 10% to 30% | Melanoma |
| Peutz-Jeghers | STK11 | 75 to 125 | 11% to 66% | GI, lung, breast, reproductive |
| Hereditary nonpolyposis colon cancer (Lynch II) | MLH1, MSH2, MSH6 | 8 to 10 | 3.7% to 10% | Colorectal, ovary, uterine, upper GI, urinary tract |
| Li-Fraumeni | TP53 | 7 | unknown | Breast, brain, adrenal |
| Familial adenomatous polyposis | APC | 4.5 | Less than 5% | Colon, upper GI, thyroid, brain |
| Ataxia telangiectasia | ATM | 8 to 9 | 1% to 5% | Breast, prostate |
| Hereditary pancreatitis | PRSS1, SPINK1 | 50 to 82 | 25% to 44% | – |
| Cystic fibrosis | CFTR | 5 | Less than 5% | – |
| Familial pancreatic cancera | 1 first-degree relative | 4.6 | – | – |
| 2 first-degree relatives | 6.4 | – | – | |
| 3 first-degree relatives | 32 | – | – | |
| aRisk determined by number of affected first-degree relatives rather than specific gene. | ||||
With the low population incidence of PDAC (lifetime risk: 1.3%), absence of biomarker screening targets, and high cost of sensitive imaging methods, the U.S. Preventive Services Task Force recommended against screening for pancreatic cancer in asymptomatic adults in 2019, reaffirming its previous conclusion in 2004 [74]. As population screening to achieve earlier detection and intervention of PDAC is not currently feasible, other approaches for this objective have been identified.
In Australia, public awareness campaigns have highlighted the often-vague symptoms of PDAC and encouraged individuals to seek medical attention early. Underscoring this point, one study found that many people who were ultimately diagnosed with PDAC were falsely reassured by the subtle, intermittent nature of their symptoms over the preceding months [75,76].
As a relatively rare cancer, many primary care providers will only see a PDAC case every few years, making it imperative to elevate awareness of early PDAC signs and symptoms among these professionals. A retrospective case-control study in primary care found that patients sought medical attention 18 times on average in the period preceding their pancreatic cancer diagnosis. PDAC was associated with 11 alarm symptoms; back pain, lethargy, and new-onset diabetes were unique features of PDAC [75,77].
Specific screening efforts in PDAC have focused on identifying high-risk individuals [48]. In 2020, both the International Cancer of the Pancreas Screening (CAPS) Consortium and the American Gastroenterological Association (AGA) published updated recommendations for the management of individuals with increased risk of pancreatic cancer. Screening is recommended in first-degree relatives of patients with pancreatic cancer who have one or more other genetically related relative with the diagnosis. Screening should be considered in anyone with genetic syndromes associated with increased risk of PDCA. These include persons with Peutz-Jeghers syndrome, hereditary pancreatitis, or known CDKN2A gene mutation; and persons with one or more first-degree relatives with pancreatic cancer who are carriers of a germline BRCA1, BRCA2, PALB2, or ATM gene mutation (Table 5) [71;137)]. Surveillance of high-risk individuals is recommended to detect and resect early pancreatic cancer and its high-grade precursors (Table 5). No consensus was reached on whether surveillance should be performed for hereditary pancreatitis.
INTERNATIONAL CANCER OF THE PANCREAS SCREENING (CAPS) CONSORTIUM CONSENSUS ON SCREENING FOR PANCREATIC CANCER IN PATIENTS WITH INCREASED RISK FOR FAMILIAL PANCREATIC CANCER
| What is the goal of pancreatic surveillance? | ||||||
| The primary goal is to prevent the emergence of and death from pancreatic cancer by identifying and treating stage I pancreatic cancer (resected with negative margins) and pancreatic cancer precursor lesions with high-grade dysplasia (PanIN or IPMN). | ||||||
| Who should be screened? | ||||||
| ||||||
| At what agea should pancreatic surveillance begin? | ||||||
| Familial pancreatic cancer kindred | Start at 50 or 55 years of age, or 10 years younger than the youngest affected blood relative | |||||
| Mutation carriers | For CDKN2A and Peutz-Jeghers syndrome, start at 40 years of age | |||||
| For BRCA2, ATM, PALB2, BRCA1, and MLH1/MSH2, start at 45 or 50 years of age, or 10 years younger than the youngest affected first-degree relative | ||||||
| What tests and indications? | ||||||
| Indication | Interval | Test(s) | ||||
| Routine | At baseline and during follow-up |
| ||||
| Concerning abnormalities for which immediate surgery is not indicated | After 3 to 6 months | Repeat follow-up testing | ||||
| No abnormalities or only non-concerning abnormalities (e.g., pancreatic cysts without worrisome features) | After 12 months | Repeat follow-up testing | ||||
| If concerning features on imaging | Upon indication | Serum CA 19-9 | ||||
| Upon indication | Endoscopic ultrasound-guided FNA | ||||
| Upon indication | CT | ||||
| Positive FNA and/or a high suspicion of malignancy on imaging | Upon indication | Surgeryb | ||||
| ||||||
However, it is important to remember that among patients with PDAC unselected for their family history of pancreatic cancer who had a germline susceptibility gene mutation, only 10% of these patients had a family history of pancreatic cancer, and most did not have a cancer family history to suggest an inherited cancer syndrome. Because family history remains one of the best predictors of future pancreatic cancer risk, routine gene testing of patients with newly diagnosed PDAC and their families may yield significant clinical benefits [78].
Genetic counseling of patients before and after any genetic testing is essential, to provide understanding and reassurance and to avoid harm. A challenge to less restrictive testing of patients with new PDAC is there are not enough genetic counselors to provide this service; this shortage of expertise applies to other cancers as well [78].
With strong consensus that benefits outweigh harms, in 2018 the ASCO recommended germline genetic testing for patients with PDAC, even if family history is unremarkable, if an informative result could directly benefit the patient or their family members [73]. This stance was adopted in 2020 by the NCCN. Consensus has subsequently expanded.
All patients with pancreatic cancer should have germline testing and gene profiling offered as quickly as possible after diagnosis; the implications for first-line therapy and beyond are significant [79,80]. The 2020–2021 ASCO and NCCN recommendations are for all patients with PDAC to receive germline genomic testing using comprehensive gene panels for hereditary cancer syndromes, and targeted (somatic) profiling of tumor tissue using next-generation sequencing [10,11]. Patients with locally advanced or metastatic PDAC should have available tumor tissue tested for DNA mismatch repair deficiency (dMMR) and microsatellite instability-high (MSI-H) status. It is also recommended that these patients undergo testing for actionable somatic mutations, including fusions (ALK, NRG1, NTRK, ROS1), mutations (BRAF, BRCA1/2, HER2, KRAS, PALB2), and mismatch repair deficiency (dMMR).
Advances in genomic analysis of human tissue from patients with pancreatic cancer and precancer are enabling the application of DNA-based molecular approaches to early detection of risk for PDAC. Circulating micro RNAs (miRNAs) are a group of small non-coding RNAs that regulate gene expression. These micromolecules are highly stable and can be quantified in small specimens of tissue or plasma. Using plasma samples collected from prospective cohort studies, investigators systematically screened for and validated specific circulating miRNAs as biomarkers associated with pancreatic cancer risk. Three miRNA targets, identified from prediagnostic plasma samples, were associated with the development of pancreatic cancer within five years of sample collection. In addition, five other specific miRNAs were associated with pancreatic cancer risk specifically among patients older than 65 years of age [138]. If confirmed by larger studies, miRNA biomarkers may be useful in identifying individuals at risk of developing pancreatic cancer, who then would be candidates for further screening and close surveillance.
Most pancreatic cancers (approximately 70%) originate in the head of the pancreas and present with biliary obstruction leading to dark urine (49%), jaundice (49%), loss of appetite (48%), weight loss (55%), and pancreatic insufficiency (25%) [134]. Symptoms of pancreatic cancer arising in the body and tail of the pancreas are more nonspecific, such as abdominal pain, back pain, weight loss and fatigue. Pancreatic cancers typically metastasize to regional lymph nodes first, then to the liver. PDAC can also directly invade surrounding visceral organs (e.g., duodenum, stomach, colon); metastasize to any surface in the abdominal cavity via peritoneal spread where development of ascites carries an ominous prognosis; or spread to the skin as painful nodular metastases. By the time of diagnosis, 85% to 90% of patients have locally advanced tumors that have involved retroperitoneal structures, spread to regional lymph nodes, or metastasized to the liver or lung [2,13,24,81].
Early-stage pancreatic cancer is notoriously difficult to diagnose. The most common symptoms in a series of patients diagnosed with PDAC were fatigue (86%), weight loss (85%), anorexia (83%), abdominal pain (79%), epigastric pain (71%), jaundice (56%), nausea (51%), diarrhea (44%), pruritus (32%), and steatorrhea (25%) [82].
Abdominal pain, jaundice, and weight loss are nonspecific, subtle in onset, and easily attributed to other processes. Unless the healthcare provider has a high index of suspicion for the possibility of underlying pancreatic carcinoma, this can make it difficult to know when to escalate a workup, as PDAC lacks a specified diagnostic algorithm [2,24].
Development of abdominal pain, jaundice, or weight loss in the context of newly diagnosed diabetes, family history of PDAC, or history of pancreatitis should trigger inclusion of PDAC in the differential diagnosis [2]. Furthermore, past three-year onset of diabetes or ongoing hyperglycemia with significant weight loss and decreasing serum lipids should be considered a potential PDAC, even if abdominal pain or jaundice are absent, with urgent referral a priority.
As noted, pancreatic cancer-associated diabetes and pancreatic cancer cachexia are distinct paraneoplastic syndromes with clinical parameters that may alert attentive clinicians to pursue an appropriately aggressive workup [47]. The lethality of pancreatic cancer merits such an approach despite the absence of formal diagnostic guidelines in this area.
A peculiar herald sign of occult pancreatic cancer is the insidious onset of an enigmatic depression, absent abdominal pain and often accompanied by anorexia and weight loss. In some patients, depression may be the most prominent presenting symptom, possibly secondary to delayed diagnosis. In addition, although patients may not communicate it to their families, they are often aware that a serious illness of some kind is occurring in them [24]. The risk of suicide among male patients with PDAC is almost 11 times higher than the general male population. Patients who underwent resection are more likely to commit suicide, specifically in the early postoperative period [83].
The association between mood disorders, fatigue, and PDAC has been assumed secondary to the psychosocial impact of diagnosis, loss of independence, and treatment toxicity [2]. However, it is now clear that PDAC has independent detrimental effects on the brain. These symptoms, often present before a diagnosis, are collectively the greatest drivers of declines in health-related quality of life and are independently predictive of survival. Evidence points to neuroinflammatory processes and the need to rethink PDAC as a systemic disease [2].
The importance is emphasized of taking a thorough family history when seeing a new patient with pancreatic cancer. A family history of pancreatitis, melanoma, and pancreatic, colorectal, breast and ovarian cancers should be noted [11].
If a cancer syndrome is identified, at-risk relatives should be offered genetic counseling. With or without a known syndrome, individuals with a suspicious family history should be advised on risk-reducing strategies, including smoking cessation and weight loss. The possibility of screening for pancreatic and other cancers should be discussed.
Referral for genetic counseling should be considered for patients diagnosed with pancreatic cancer, especially those with a family history of cancer or who are young, those of Ashkenazi Jewish ancestry, or for whom a hereditary cancer syndrome is suspect. A free pancreatic cancer risk prediction tool, PancPRO, is available and may help determine risk [11].
Some, but not all, initial symptoms of PDAC result from a mass effect, such that pancreatic tumor location influences the stage of disease progression when symptoms appear [13].
Abdominal pain is the most common symptom, usually insidious in onset and often present for one to two months at the time of presentation, the pain is often severe, and unrelenting in nature. The typical gnawing, visceral quality of pain is generally epigastric, radiating to the sides and/or straight through to the back; some patients may describe the pain as originating in the back. Nighttime pain is often the predominant complaint. Some patients note increased pain after eating and worsened pain when lying flat [24,81]. Rarely, acute pain develops when an episode of acute pancreatitis results in tumor occlusion of the main pancreatic duct [84].
While roughly one-third of patients may not have pain at the time of initial presentation, all patients will develop pain at some point [24]. Pancreatic cancer is one of the most painful malignancies, and effective pain control is extremely important [85]. This issue will be discussed in detail later in this course.
The most characteristic sign of tumor in the pancreatic head is obstructive jaundice, for which patients may seek medical attention before their tumor grows large enough to cause abdominal pain (and thus, a somewhat better prognosis). These patients usually notice a darkening of their urine and/or lightening of their stools before they or their families notice the change in skin pigmentation. Jaundice secondary to a tumor in the body or tail of the pancreas typically occurs at a later stage and may be secondary to liver metastases of PDAC [2,84].
Pruritus can accompany and often precedes obstructive jaundice. If present, it is often the patient's most distressing symptom [24].
A characteristic feature of pancreatic cancer, significant weight loss may be related to cancer-associated anorexia and/or subclinical malabsorption from pancreatic exocrine insufficiency caused by pancreatic duct obstruction. Nausea and early satiety from gastric outlet obstruction and delayed gastric emptying from the tumor can contribute to weight loss [24]. Significant weight loss is a symptom of cachexia.
Pancreatic cancer cachexia is a multifactorial paraneoplastic syndrome characterized by a loss of skeletal muscle mass, commonly associated with adipose tissue wasting and anorexia, fatigue, and reduced exercise tolerance. Cachexia develops in approximately 80% of patients with PDAC, in whom the syndrome is typically present at diagnosis and responds poorly to therapeutic interventions [47,86].
Pancreatic cancer leads to the development of cachexia through a combination of distinct factors that explain its high prevalence and clinical importance in this disease [86]:
Systemic factors, including metabolic changes and pathogenic signals related to PDAC tumor biology
Factors resulting from the disruption of the digestive and endocrine functions of the pancreas
Factors related to the close anatomic and functional connection of the pancreas with the gut
The initial assessment can uncover additional diagnostic clues. Undiagnosed diabetes leads to symptoms of glucose intolerance (e.g., polyuria, polydipsia). PDAC can interfere with production of digestive enzymes by the pancreas (pancreatic exocrine insufficiency) and with the ability to break down food and absorb nutrients (malabsorption) in some patients. This malabsorption causes bloating, gas, and a watery, greasy, and/or foul-smelling diarrhea, leading to weight loss and vitamin deficiencies [81].
While long-standing diabetes is a risk factor for later development of PDAC, new-onset hyperglycemia or diabetes has been identified in most patients at diagnosis of otherwise asymptomatic PDAC. Deregulation in glucose homeostasis is often accompanied by changes in subcutaneous adipose tissue. Both represent paraneoplastic syndromes caused by the underlying PDAC [2].
This research is among the most important knowledge advances in PDAC in the past decade. In addition to metabolic deregulation, the pre-diagnostic soft tissue changes and symptoms of cachexia have profound implications for screening, early diagnosis, treatment selection, and patient prognosis [2].
Tumors can also grow locally into the duodenum (proximal for the head of the pancreas, distal for the body and tail of the pancreas) and result in an upper gastroduodenal obstruction [13]. Tumor in the body or tail of the pancreas may cause splenic vein obstruction, resulting in splenomegaly, gastric and esophageal varices, and gastrointestinal hemorrhage [81].
Clinical signs of PDAC during physical examination include jaundice, pruritus, steatorrhea, and vascular issues [2,24,82,84]. Healthcare professionals can usually recognize clinical jaundice when total bilirubin reaches 2.5–3 mg/dL. Patients and their families do not usually notice clinical jaundice until total bilirubin reaches 6–8 mg/dL. Patients with jaundice may have a palpable gallbladder (i.e., Courvoisier sign). As noted, patients with clinical jaundice may have skin excoriations from unrelenting pruritus. If the pancreas has lost the ability to secrete fat-digesting enzymes or if the main pancreatic duct is blocked, steatorrhea will develop.
Migratory thrombophlebitis (i.e., Trousseau syndrome) and venous thromboembolism, reflecting the hypercoagulable state that frequently accompanies pancreatic cancer, may be the initial clinical sign. Thromboembolic events (both venous and arterial) are especially prevalent in advanced disease, and thromboembolic complications occur more commonly with tumors in the pancreatic tail or body.
Multiple arterial emboli resulting from nonbacterial thrombotic endocarditis may be the presenting sign of PDAC. Marantic endocarditis (also known as nonbacterial thrombotic endocarditis) may develop in patients with pancreatic cancer and possibly mimic subacute bacterial endocarditis.
Metastatic disease most commonly affects the liver, peritoneum, lungs, and less frequently, bone [24,84]. Patients presenting with or developing advanced intra-abdominal disease may have ascites, a palpable abdominal mass, hepatomegaly from liver metastases, or splenomegaly from portal vein obstruction. Subcutaneous metastases (termed Sister Mary Joseph nodules) in the paraumbilical area signify advanced disease; pancreatic cancer is the origin of a cutaneous metastasis to the umbilicus in 7% to 9% of cases [24,84]. A metastatic mass in the rectal pouch may be palpable on rectal examination (Blumer shelf). As a metastatic node, left supraclavicular lymphadenopathy may be palpable, while other nodes in the cervical area may also be involved.
Routine laboratory tests are often abnormal but nonspecific for PDAC. Common abnormalities include an elevated serum bilirubin and alkaline phosphatase levels, and presence of mild anemia [84].
Patients presenting with jaundice or epigastric pain should be evaluated with complete blood count, blood chemistry panel, and liver function tests to help assess the extent of cholestasis (bilirubin), liver metastasis (alkaline phosphatase), hepatitis (aminotransferases), and nutritional status (albumin, prealbumin). With epigastric pain, serum lipase should be measured to evaluate for acute pancreatitis [2].
Differential diagnosis before imaging and biopsy includes acute/chronic pancreatitis, cholangitis, cholecystitis, choledochal cyst, peptic ulcer disease, cholangiocarcinoma, and gastric cancer [85]. Unlike pancreatic exocrine tumors, the symptoms of pancreatic neuroendocrine tumors are distinctly related to excessive secretion of hormones such as insulin, glucagon, gastrin, somatostatin, and vasoactive peptide, resulting in hypoglycemia, hyperglycemia, and GI disturbances such as peptic ulcer and diarrhea.
When diagnostic imaging in a patient presenting with obstructive jaundice reveals an abnormality of the region of the pancreas, the differential diagnosis is usually that of a soft-tissue mass involving the head of the pancreas, causing common bile-duct obstruction. Imaging studies help characterize such lesions as either extrapancreatic, cystic or solid pancreatic mass lesions [139]. Extrapancreatic masses, which can be difficult to distinguish from intrinsic pancreatic lesions, include those caused by regional lymphadenopathy and ampullary, duodenal, and bile duct neoplasms. Cystic pancreatic masses include retention cysts, papillary neoplasms, and mucinous cystic or serous cystic neoplasms. When imaging shows a solid pancreatic mass, the possibilities include exocrine pancreatic cancer (PDAC), pancreatic neuroendocrine tumor, autoimmune pancreatitis, metastatic cancer, and lymphoma [139].
It is not possible to reliably diagnose a patient with pancreatic cancer based on symptoms and signs alone. Abdominal imaging is used in the diagnostic and staging workup of a patient with suspected PDAC. Additional testing is based on the initial findings, the patient's clinical presentation and risk factors [2].
Accurate PDAC detection and staging at the time of presentation carries substantial implications for appropriate recommendation to patients of the most suitable treatment option, thus maximizing the survival benefit for patients in whom complete resection can be achieved and minimizing the morbidity from unnecessary laparotomy or major surgery in patients with high risk of residual disease following resection. The accuracy critically depends on the appropriate imaging protocol and radiologist experience [2,87]. As such, decisions about diagnosis, resectability, and management of pancreatic cancer should involve multidisciplinary consultation at high-volume centers [11].
Multidetector computed tomography (MDCT) angiography with intravenous (IV) contrast is the preferred imaging for initial evaluation of suspected PDAC. The Pancreatic CT Protocol standardizes its use, making MDCT highly accurate for assessing tumor extent, vascular invasion, and distant metastases [11,16,88,89]. The degree of contact between the tumor and local blood vessels (i.e., uninvolved, abutted, or encased) is used to define the most optimal initial treatment [134]. The NCCN recommends that MDCT angiography should also cover the chest and pelvis for complete staging [11].
MDCT is 77% accurate in predicting resectability and 93% accurate in predicting unresectability [85]. MDCT may be superior to magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) in vascular enhancement of a PDAC, the most important parameter of resectability. However, MDCT is inferior to MRI/MRCP in depicting isodense tumors or tumors smaller than 1.5 cm in size [54].
Abdominal MRI/MRCP with IV contrast also employs a standard multiphase protocol in PDAC, with efficacy comparable to MDCT in preoperative evaluation and assessment of vascular invasion. The sensitivity of MRI/MRCP in detecting liver metastases is nearly 100% (vs. 80% with MDCT) [81,85].
Selection of initial MDCT or MRI/MRCP is typically based on local availability and expertise [81,85]. Following initial MDCT, MRI/MRCP is used when PDAC is highly suspected but negative on MDCT, for characterizing small or indeterminate pancreatic and hepatic tumors, and in patients with severe allergy to iodinated IV contrast material used in MDCT [54,81,85].
With endoscopic retrograde cholangiopancreatography (ERCP), contrast dye is injected into the biliary ducts and pancreatic duct with an endoscope, and the level of obstruction is delineated. In some case, placement of a biliary stent can help relieve symptoms of jaundice [85]. Patients with obstructive jaundice may have ERCP as the first diagnostic procedure [81].
Transabdominal ultrasonography is useful in initial screening of patients who present with possible obstructive jaundice and can rapidly and accurately assess for biliary obstruction. However, definitive diagnosis requires other imaging [24].
Endoscopic ultrasonography is superior to MDCT in detecting solid pancreatic lesions less than 2 cm in size, with accuracy of about 92% [54]. Endoscopic ultrasonography-guided fine-needle core biopsy (preferred over fine-needle aspiration) is recommended to obtain a histologic diagnosis and to provide material for molecular testing [134].
With the restricted field of view, endoscopic ultrasonography is complimentary to MDCT, but it should be used before other imaging options if no pancreatic mass is evident on MDCT. Endoscopic ultrasonography is also valuable in detecting tumor involvement of blood vessels or lymph nodes [11,89].
Positron-emission tomography (PET) imaging alone does not offer added advantages to MDCT. Combining PET with CT (PET/CT) is a more recent development that may enhance the detection of occult metastases in pancreatic cancer. The NCCN guidelines consider PET/CT an evolving technology; its role in the diagnosis of PDAC is not yet established [11].
Endoscopic ultrasound-guided biopsy is preferable to a CT-guided biopsy in patients with non-metastatic disease because of better diagnostic yield, safety, and lower risk of peritoneal seeding when compared with the percutaneous approach. Biopsy proof of malignancy is not required before surgical resection, and a non-diagnostic biopsy should not delay surgical resection when the clinical suspicion for pancreatic cancer is high [11]. However, when histologic confirmation of a pancreatic cancer diagnosis is required, as in certain situations, endoscopic ultrasonography-guided fine-needle core biopsy is the best modality for obtaining a tissue diagnosis.
A pathologic diagnosis is indicated to confirm PDAC in locally advanced or metastatic disease, before neoadjuvant therapy, and in atypical presentations in which differential diagnosis is needed with other pancreatic masses (e.g., pancreatitis, lymphoma, tuberculosis). If a biopsy does not confirm malignancy, it should be repeated at least once [16].
The difficulty of diagnosing PDAC in patients with underlying chronic pancreatitis is noteworthy. In such cases, all typical imaging methods may show abnormalities that do not differentiate between PDAC and chronic pancreatitis, and carbohydrate antigen 19-9 (CA19-9) may be similarly elevated in pancreatitis. These patients may require combined multiple imaging modalities, close follow-up, serial imaging studies, and in some cases, empiric resection to diagnose an underlying pancreatic carcinoma [24].
CA19-9 is a sialylated Lewis A blood group antigen, commonly expressed and shed in benign and malignant pancreatic and biliary disease. Although not sufficiently sensitive or specific for routine screening, CA19-9 is the most clinically useful biomarker in PDAC, with sensitivity (79% to 81%) and specificity (82% to 90%) in symptomatic patients. A normal serum level is 37 U/mL [90].
Preoperative CA19-9 provides important prognostic information. Levels <100 U/mL imply likely resectable disease, while levels >100 U/mL suggest unresectablity or metastatic disease. Fewer than 4% of patients with levels >300 U/mL have resectable tumors [24,90].
In one study, patients with preoperative CA19-9 levels <37 U/mL showed longer median survival (22 to 40 months) than patients with levels >37 U/mL (7 to 30 months). Post-treatment changes (two to five weeks post-resection; six to eight weeks post-chemotherapy) from baseline may predict overall survival [90,91].
CA19-9 is a useful biomarker for monitoring treatment response. Post-operative CA19-9 levels of <37 U/mL, <200 U/mL, and >500 U/mL were associated with three-year survival rates of 49%, 38%, and 0%, respectively. Post-chemotherapy CA19-9 decreases of ≥20% predicted prolonged disease-free survival and overall survival [90,91].
Around 5% to 10% of the population lacks the enzyme necessary to produce CA19-9; monitoring pancreatic cancer with this marker will not be possible in these individuals [24]. Biliary obstruction also stimulates the secretion of CA19-9. Hyperbilirubinemia is associated with elevated CA19-9 and false positivity in patients with obstructive jaundice. Following the treatment of obstruction, re-evaluation of CA19-9 should improve its diagnostic utility [92].
The NCCN recommends measurement of serum CA19-9 levels after neoadjuvant treatment, prior to and immediately following surgery before adjuvant therapy, and in surveillance. The importance is stressed of obtaining CA19-9 immediately before a therapeutic intervention to have an accurate baseline from which to follow response [11].
When a solid tissue mass lesion of the pancreas is detected on MDCT (with or without additional imaging), it is reasonable to conclude that a neoplasm is present and is most likely malignant PDAC. After a probable diagnosis of pancreatic cancer is made, the next step is the staging evaluation to establish disease extent and resectability. Unlike many other cancers, imaging is the primary means through which the stage of pancreatic cancer is determined [11].
Using initial MDCT (with or without additional imaging), two different systems are involved [11,93]:
American Joint Committee on Cancer (AJCC) TNM staging system, to assess tumor status/extent (T), lymph nodes (N), and metastasis (M)
NCCN guideline to characterize resectable, borderline resectable, or locally advanced disease
The AJCC system (Table 6) is used for staging PDAC in two contexts [16,94]:
Clinical staging of all patients with imaging assessment of tumor size and extension, nodal involvement, and distant disease spread
Pathologic staging of tissue specimens obtained during resection for presence of viable tumor cells
AMERICAN JOINT COMMISSION ON CANCER EXOCRINE PANCREATIC CANCER TNM STAGING
| Category | Criteria |
|---|---|
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ, including high-grade PanIN (PanIN-3) and IPMN, ITPN, or MCN with high-grade dysplasia |
| T1 | Tumor ≤2 cm in greatest dimension |
| T1a | Tumor ≤0.5 cm in greatest dimension |
| T1b | Tumor >0.5 and <1 cm in greatest dimension |
| T1c | Tumor 1–2 cm in greatest dimension |
| T2 | Tumor >2 and ≤4 cm in greatest dimension |
| T3 | Tumor >4 cm in greatest dimension |
| T4 | Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery, regardless of size |
| Regional lymph nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in one to three regional lymph nodes |
| N2 | Metastasis in four or more regional lymph nodes |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| IPMN = intraductal papillary mucinous neoplasm; ITPN = intraductal tubulopapillary neoplasm; MCN = mucinous cystic neoplasm; PanIN = pancreatic intraepithelial neoplasia | |
Clinical staging identifies the primary tumor and its vessel involvement, enlarged or suspicious lymph nodes, and metastatic disease sites. TNM staging provides important prognostic information (Table 7) but does not assess whether the PDAC tumor is amenable to surgical resection [54,94].
Complete resection is the only potentially curative treatment for PDAC, but fewer than 20% of patients presenting with PDAC have localized and easily resectable tumors, and noncurative resections provide no survival benefit. Thus, accurate assessment of resectability is crucial [24,87,89].
The NCCN guideline classes PDAC resectability into the following clinical stages [11]:
Stage 1: Resectable
Stage 2: Borderline resectable (i.e., tumors that are involved with nearby structures so as to be neither clearly resectable nor clearly unresectable with a high chance of removal of all macroscopic disease)
Stage 3: Locally advanced (i.e., tumors that are involved with nearby structures to an extent that renders them unresectable despite the absence of metastatic disease)
Stage 4: Metastatic (i.e., non-resectable)
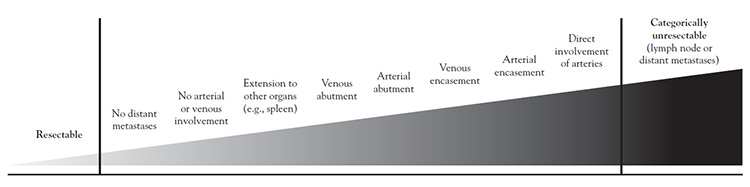
Localized PDAC falls on a spectrum from high to low resectability, determined by the extent of vessel contact and whether the involvement is arterial or venous (Figure 1) [11,54,84,87,89,95]. Major peripancreatic vessels include the superior mesenteric vein and artery, portal vein, common hepatic artery, and celiac artery. Tumor contact can be characterized as encasement (≥180 degrees of the vessel circumference), abutment (<180 degrees of the circumference), or direct involvement (absence of fat plane between tumor and vessel).
In the past, vascular infiltration by PDAC was considered unresectable, but surgical advances have increased the number of patients with initial borderline resectable or locally advanced disease who can undergo resection. In general, venous abutment or encasement is usually borderline resectable as long as the venous segment is reconstructable. Arterial reconstruction is substantially more difficult and riskier than venous reconstruction with comparable tumor contact.
Based on PDAC clinical status of resectable, borderline resectable, locally advanced, or metastatic disease, additional considerations and therapeutic approaches will be undertaken. The time-urgency between the first availability of full imaging findings, multidisciplinary evaluation, the diagnostic and staging workup, discussion with the patient of available treatment options, and treatment initiation cannot be overstated in this aggressive malignancy.
As mentioned, the initial imaging workup of PDAC confirms the diagnosis, searches for evidence of metastases, and classifies nonmetastatic PDAC into resectable, borderline resectable, or locally advanced disease based on the involvement of surrounding arterial (superior mesenteric artery, common hepatic artery, and celiac axis) and venous (superior mesenteric vein or portal vein) structures, and other nearby organs and lymph nodes [96].
On average, 10% to 20% of patients initially present with "up-front" resectable PDAC. However, an increasing number of patients with initial borderline resectable or locally advanced disease are eligible for surgical resection as a result of neoadjuvant (i.e., before resection) therapies which may downstage the tumor, and advances in surgical technique, such as venous reconstruction in a vascular infiltration formerly considered unresectable [2].
In all therapeutic decisions, multidisciplinary collaboration to formulate treatment planning and disease management that incorporates patient preferences and available support, their comorbidity profile, symptom burden, and performance status should be the standard of care [6,7,10].
Performance status is an important indicator of general well-being and the ability to perform activities of daily living in patients with cancer and is frequently assessed in both clinical and research settings. Performance status is repeatedly shown to predict important clinical outcomes, including quality of life, chemotherapy toxicity, response to chemotherapy, terminal illness, progression-free survival, and overall survival in patients with cancer [97].
The Karnofsky Performance Status tool has been used for this purpose, but PDAC guidelines and randomized controlled trials now solely employ the Eastern Cooperative Oncology Group Performance Status (ECOG) scale (Table 8) [97]. For instance, some chemotherapies are indicated solely for patients with good ECOG performance status (0 or 1).
EASTERN COOPERATIVE ONCOLOGY GROUP (ECOG) PERFORMANCE STATUS SCALE
| Score | Definition | |||
|---|---|---|---|---|
| 0 |
| |||
| 1 |
| |||
| 2 |
| |||
| 3 |
| |||
| 4 |
| |||
| 5 | Deceased |
Baseline functional status and comorbidity profile should be carefully evaluated, because both have major implications for a person's ability to tolerate therapy. Performance status is consistently identified as a prognostic factor for people with pancreatic cancer. It is also an important determinant in treatment selection; some patients with up-front resectable PDAC may be physically weakened by weight loss and cachexia to an extent that places them at high risk of serious complications or mortality from definitive surgery. Performance status also helps predict chemotherapy toxicity, which can determine the treatment approach for patients with performance status 0 to 1 (multi-agent regimens) or performance status ≥2 (e.g., single-agent gemcitabine) [8].
Similarly, the comorbidity profile can influence the choice of chemotherapy, such as avoiding fluoropyrimidine-based regimens in patients with a known history of uncontrolled coronary artery disease. Nonetheless, performance status and comorbidities alone should not be used simply to rule in or out patients for treatment. For instance, disease control of comorbidities, such as controlled type 2 diabetes, can indicate that patient benefit from treatment may outweigh risks associated with poorly controlled comorbid diabetes [8].
Treatment approaches for PDAC include surgical resection, chemotherapy, radiation therapy, and combined regimens (chemoradiation therapy). Chemotherapy is the backbone of pancreatic cancer treatment and is employed in all stages of PDAC. This includes preoperative neoadjuvant therapy (resectable or borderline resectable), postoperative adjuvant therapy, and first-line or subsequent therapy in locally advanced, metastatic, and recurrent disease [11]. Most patients present with disease too far advanced to benefit from surgery or that surgical resection alone will not provide a survival advantage over what can be achieved with supportive care. Chemotherapy and radiation therapy also have a role in palliation, as will be discussed in a later section [99].
Curative surgical approaches for resectable pancreatic cancer are well-established. In contrast, the pace of new U.S. Food and Drug Administration (FDA) approvals and/or phase III evidence continue to make chemotherapy, molecular-targeted therapy, radiation, and chemoradiotherapy approaches a fluid, evolving area, requiring frequent updating and revisions in multidisciplinary clinical practice guidelines for pancreatic cancer treatment. Many potential treatment approaches lacking phase III or prospective evidence are being addressed, with publication of trial results awaited [2].
For patients with resectable or borderline resectable PDAC, neoadjuvant therapy consists of chemotherapy with or without radiation therapy before radical pancreatic resection [99]. Radical pancreatic resection may include Whipple procedure (pancreaticoduodenal resection) or total pancreatectomy when necessary for adequate margins. Distal pancreatectomy is indicated for tumors of the body and tail of the pancreas. Following surgical resection, patients may receive postoperative chemotherapy or postoperative chemoradiation therapy [99].
Chemotherapy with or without targeted therapy is recommended for patients with locally advanced PDAC [99]. For patients without metastatic disease, this should be followed by chemoradiation therapy. If removal is a possibility, radical pancreatic resection may be attempted. Palliative surgery options include surgical biliary and/or gastric bypass, percutaneous radiologic biliary stent placement, or endoscopic biliary stent placement.
Treatment of metastatic or recurrent PDAC is limited to chemotherapy with or without targeted therapy [99]. Palliative approaches should be used whenever available and feasible to improve patient comfort and quality of life.
Selecting patients for surgery should be based on the probability of cure as determined by resection margins. Other factors include comorbidities, overall performance status, and age. Pancreaticoduodenectomy and distal and total pancreatectomy are curative resection options based on the location, size, and locally invasive aspects of the tumor. Each has its own set of perioperative complications and risks, which should be considered by the surgical team and discussed with the patient [24].
Mortality rates from resection have fallen significantly, but morbidity remains common and interferes the delivery of adjuvant therapy in up to 40% of patients. The NCCN recommends that patients seek out high-volume centers performing more than 15 to 20 resections annually, with multidisciplinary expertise to optimize their treatment plan and increase opportunities for clinical trial participation [2].
The only curative treatment for PDAC is radical surgery, but potential cure is only possible with a microscopically negative resection margin (R0). Macroscopic (R2) and microscopic (R1) margin infiltration have survival trends similar to patients without surgery. R0 is a minimum >1 mm distance of viable tumor cells from the resection margin, R1 is ≤1 mm distance. A retrospective analysis of 44,852 patients with PDAC reported median survival of 19.7 months following R0, 14.3 months following R1, and 9.8 months with R2 resections compared with 10.3 months without surgery [100]. An incomplete tumor resection imposes morbidity risks without benefit to the patient, and the aim of resection is to obtain microscopically negative margins (R0) [101].
Tissue specimens obtained during resection are examined. During resection, lymphadenectomy is performed, including at least 15 lymph nodes, which are likewise examined as part of pathologic staging [16].
With surgical advances and greater use of adjuvant therapies, long-term cancer survival outcomes following resection were anticipated to improve over time [102]. However, in 1,147 pancreatic resections performed over three decades at the Memorial Sloan Kettering Cancer Center, a lack of progress in long-term survival was reported. Although patients treated between 2000 and 2009 had lower rates of operative mortality and greater one-year survival, for patients treated in the 1980s, 1990s, and 2000s, the median survival was 23.2, 25.6, and 24.5 months, respectively [103]. The five-year survival rates were 17%, 20%, and 8%, respectively. These data underscore the need for earlier detection and more effective systemic therapies [102].
Pancreaticoduodenectomy (Whipple Procedure)
Used for tumors in the pancreatic head or periampullary region, the conventional Whipple procedure involves removal of the pancreatic head, duodenum, gallbladder, and the antrum of the stomach, with surgical drainage of the distal pancreatic duct and biliary system, usually through anastomosis to the jejunum. The primary reason for removing so much of the intra-abdominal structures is that they all share a common blood supply [24,102].
The former high morbidity and mortality rates of Whipple have declined with the greater experience of a more limited number of surgeons who regularly perform the procedure in high-volume centers [102]. Common morbidities include delayed gastric emptying in roughly 25% of patients, which may require nasogastric decompression and a longer hospital stay. Pancreatic anastomotic leak can be treated with adequate drainage. Postoperative abscesses are not uncommon [24].
With operative mortality associated with Whipple decreasing from around 25% in the 1970s to less than 2% at high-volume centers in the 2010s, the focus has shifted from surviving the operation to surviving the cancer [104].
Distal Pancreatectomy
Distal pancreatectomy is a procedure for tumors in the pancreatic body or tail. It has a lower mortality than standard Whipple, but its use in curative resection is limited; with tumors in this location seldom causing bile duct obstruction, most patients present at a later stage with unresectable disease. The procedure involves resection of the distal pancreas containing the tumor with splenectomy and over-sewing of the distal pancreatic duct. Complications involve pancreatic stump leak, hemorrhage, and endocrine insufficiency. Laparoscopic exploration should precede attempted resection, because occult peritoneal metastases are common [16,24].
Total Pancreatectomy
Total pancreatectomy, the least commonly performed procedure with the highest associated mortality (8.3%), may be needed to achieve an R0 resection margin for tumors in the neck of the pancreas, especially with extension into the body or tail, and in multifocal PDAC. Total pancreatectomy may be an option to pancreatic anastomosis in highly selected patients with a high-risk pancreas (small pancreatic duct) and obese patients with pancreatic fat infiltration. The metabolic consequences of permanent exocrine insufficiency and diabetes have a detrimental impact on quality of life and long-term survival [16,24,102].
Vascular involvement has traditionally been a formal contraindication to resection. With recent advances, venous resection and reconstruction can achieve R0 resection with similar overall survival and morbidity compared to surgery without venous resection. However, arterial resection during Whipple is associated with increased mortality and morbidity (bowel ischemia, hemorrhage, thrombosis) and is generally not recommended [16].
Progress in neoadjuvant therapies may downstage some tumors with arterial invasion to borderline resectable or resectable disease, making resection more achievable. Despite these advancements, it is currently accepted that arterial reconstruction is only appropriate in highly selected patients in high-volume centers with surgeons who are familiar with the advanced techniques required for reconstruction [16].
Total pancreatectomy should be considered in patients with locally advanced tumors who undergo pancreatectomy with arterial resection and reconstruction [16].
In most patients with jaundice, early resection without biliary drainage is preferred. Preoperative drainage is indicated in patients with cholangitis or with obstructive jaundice scheduled for neoadjuvant therapy. Endoscopic retrograde placement of a fully covered metal stent is preferred. Endoscopic ultrasonography-guided stent placement is an effective and safe alternative [16].
As mentioned, the backbone of PDAC treatment is chemotherapy. Most patients present with advanced disease, and even those who undergo resection benefit from adjuvant chemotherapy. Chemotherapy is also used as neoadjuvant therapy and in metastatic disease with first-line or second-line indications [11].
Until recently, chemotherapies found effective in other GI cancers were applied to patients with advanced PDAC; the few agents showing any response became adjuvant therapies in localized PDAC. The near-futility in effective chemotherapy and redundancy in agents used in localized and metastatic PDAC reflects the pathologic complexity of this cancer and its profound resistance to cytotoxic therapies [2].
Since 2010, chemotherapy effectiveness has improved with the introduction of combination regimens, the identification of patients in whom mutational status conferred improved response to existing chemotherapies, and the introduction of novel compounds explicitly targeting mutational-related advanced PDAC.
In addition to single chemotherapy agents, the FDA has approved regimens of these agents, including FOLFIRINOX (consisting of folinic acid [also referred to as leucovorin], fluorouracil [5-FU], irinotecan [IRN], and oxaliplatin [OX]) (Table 9) [3,24,80,99]. Available chemotherapies are associated with acute and delayed toxicities, some of which can be dose-limiting (Table 10). Table 11 summarizes the 2021 NCCN guideline for chemotherapy and chemoradiotherapy in PDAC.
CHEMOTHERAPY PROTOCOLS IN PANCREATIC CANCER
| Drug | Dose and Route | Administration | Given on Days | ||||
|---|---|---|---|---|---|---|---|
| |||||||
| Gemcitabine | 1,000 mg/m2 IV | Dilute in 250 mL NS (concentration ≤40 mg/mL), administered over 30 minutes. | Days 1, 8, and 15 | ||||
| |||||||
| Gemcitabine | 1,000 mg/m2 IV | Dilute in 250 mL NS (concentration ≤40 mg/mL), administered over 30 minutes. | Days 1, 8, and 15 | ||||
| Capecitabinea | 830 mg/m2 per dose oral | Twice daily (total 1,660 mg/m2 per day), 12 hours apart. Swallow with water within 30 minutes post-meal. | Days 1 through 21 | ||||
| |||||||
| Oxaliplatinb | 85 mg/m2 IV | Dilute in 500 mL D5W, administer over 2 hours (before leucovorin). Shorter schedules (e.g., 1 mg/m2 per minute) appear safe. | Day 1 | ||||
| Leucovorin | 400 mg/m2 IV | Dilute in 250 mL normal saline or D5W, administer over 2 hours (after oxaliplatin). | Day 1 | ||||
| Irinotecanc | 150 mg/m2 IV | Dilute in 500 mL normal saline or D5W, administer over 90 minutes concurrent with the last 90 mins of leucovorin infusion, in separate bags, using Y-line connection. | Day 1 | ||||
| Fluorouracil | 2,400 mg/m2 IV | Dilute in 500–1,000 mL 0.9% normal saline or D5W, administered as continuous IV infusion over 46 hours.d | Day 1 | ||||
| |||||||
| Oxaliplatinb | 85 mg/m2 IV | Dilute in 500 mL D5W, administer over 2 hours (before leucovorin). Shorter schedules (e.g., 1 mg/m2 per minute) appear safe. | Day 1 | ||||
| Leucovorin | 400 mg/m2 IV | Dilute in 250 mL normal saline or D5W, administer over 2 hours (after oxaliplatin). | Day 1 | ||||
| Irinotecanc | 150 mg/m2 IV | Dilute in 500 mL normal saline or D5W, administer over 90 minutes concurrent with the last 90 mins of leucovorin infusion, in separate bags, using Y-line connection. | Day 1 | ||||
| Fluorouracil | 400 mg/m2 IV bolus | Give undiluted (50 mg/mL) as a slow IV push over 5 minutes (immediately after leucovorin). | Day 1 | ||||
| Fluorouracil | 2400 mg/m2 IV | Dilute in 500–1,000 mL 0.9% normal saline or D5W, administer as continuous IV infusion over 46 hours (immediately after IV bolus).d | Day 1 | ||||
| |||||||
ACUTE AND DELAYED CHEMOTHERAPY TOXICITIESa
| Agent | Acute Toxicities | Delayed Toxicities | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fluorouracil |
|
| ||||||||
| Capecitabine | Nausea and vomiting |
| ||||||||
| Gemcitabine |
|
| ||||||||
| Irinotecan | Diarrhea |
| ||||||||
| Oxaliplatin |
|
| ||||||||
| Paclitaxel | Hypersensitivity reactions |
| ||||||||
| aDose-limiting toxicities are bold-faced. | ||||||||||
NCCN TREATMENT SUMMARY FOR PDAC
| Strength of Recommendation/Evidence | Regimen | Notesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjuvant stage 1 (resectable) | |||||||||||
| Category 1 |
| – | |||||||||
| Category 2A |
| Chemoradiation should follow induction chemotherapy, with or without subsequent chemotherapy | |||||||||
| Category 2B | Capecitabine | – | |||||||||
| Neoadjuvant stage 1/2 (resectable or borderline resectable) | |||||||||||
| Category 2A | Gemcitabine/paclitaxel NAB | – | |||||||||
| Category 2B |
| – | |||||||||
| Stage 3 (locally advanced) | |||||||||||
| Category 1 | Gemcitabine | Preferred for patients with poor ECOG PS (≥2) | |||||||||
| Category 2A |
|
| |||||||||
| Category 2B |
| – | |||||||||
| Stage 4 (metastatic) | |||||||||||
| Category 1 |
| – | |||||||||
| Category 2A |
|
| |||||||||
| Category 2B |
| – | |||||||||
| Second-line therapy | |||||||||||
| Category 1 |
| – | |||||||||
| Category 2A | Gemcitabine fixed-dose rate | Fixed-dose rate gemcitabine is a category 2B recommendation for patients with poor ECOG PS (≥2) | |||||||||
| Category 2B |
| – | |||||||||
| Strength of Recommendation Definitions | |||||||||||
| Category | Definition | ||||||||||
| 1 | Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate. | ||||||||||
| 2A | Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate. | ||||||||||
| 2B | Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate. | ||||||||||
| |||||||||||
Fluoropyrimidines
Fluorouracil is a fluorinated (fluoro)-pyrimidine antimetabolite that inhibits thymidylate synthase and interferes with RNA synthesis and function, with some effect on DNA.
Capecitabine is an oral fluoropyrimidine that undergoes hepatic hydrolysis to form fluorouracil. The final enzyme, thymidine phosphorylase, is present at higher levels in tumor tissue, providing better selectivity and tolerability.
Gemcitabine is a pyrimidine antimetabolite that inhibits DNA polymerase and ribonucleotide reductase, which in turn inhibit DNA synthesis, blocks DNA replication and several forms of DNA repair [3,24,80,99].
Erlotinib
Erlotinib is a human epidermal growth factor receptor type 1/epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor. EGFR is expressed on the cell surface of normal cells and cancer cells. Erlotinib inhibits intracellular phosphorylation, which prevents further downstream signaling, resulting in cell death [3,24,80,99].
Paclitaxel
Paclitaxel protein bound is a microtubular inhibitor (albumin-conjugated formulation) and a natural taxane that prevents depolymerization of cellular microtubules, which results in DNA, RNA, and protein synthesis inhibition [3,24,80,99].
Irinotecan Liposomal
Irinotecan and its active metabolite SN-38 bind reversibly to the topoisomerase-1 DNA complex and prevent re-ligation of the single-strand breaks, leading to exposure time-dependent double-strand DNA damage and cell death. Irinotecan liposomal is used in combination with fluorouracil and leucovorin [3,24,80,99].
Platinum agents (e.g., cisplatin, oxaliplatin) and olaparib are recommended in patients with mutation in DNA damage repair (DDR) genes by the NCCN. DDR mutations are present in up to 24% of PDACs, most commonly BRCA1/2 and PALB2. Germline BRCA1/2 mutations (gBRCAm) affect approximately 7% of patients with PDAC [108]. DDR genes encode for proteins in the homologous repair pathway and DNA double-stranded break repair; thus, mutations may be more sensitive to further DNA damage [99].
Cisplatin inhibits DNA synthesis by the formation of DNA cross-links; denatures the double helix; covalently binds to DNA bases; and disrupts DNA function. Oxaliplatin is an alkylating agent. Following intracellular hydrolysis, the compound binds to DNA, forming cross-links that inhibit DNA replication and transcription, resulting in cell death [24,99].
PDACs with DDR mutations demonstrate improved responses to platinum-based therapies, and patients with advanced PDAC showed significantly improved median overall survival (22 months vs. 9 months) compared with nonplatinum therapy [96].
Poly (ADP-ribose) polymerase (PARP) inhibition has been posited to act synergistically with BRCA1/2 mutations by inhibiting single-stranded break repair, causing an accumulation of DNA damage and tumor-cell death [99,109]. Olaparib is a PARP inhibitor FDA-approved for PDAC with gBRCAm as maintenance therapy to sustain a progression-free state during platinum-based chemotherapy in metastatic PDAC [96].
The NCCN expands the use of olaparib to PDAC with gPALB2m. There are calls to expand these agents to PDACs with somatic DDR mutations [108].
The approved indications for the following agents are biomarker-defined, rather than by tumor site (e.g., pancreatic).
Pembrolizumab
Pembrolizumab is indicated in patients with microsatellite-instability-high (MSI-H) or dMMR mutations. Immune checkpoint inhibitors (ICIs) have efficacy in solid tumors with a high tumor mutational burden, and MSI-H or dMMR mutation solid tumors are associated with high tumor mutational burden. The ICI pembrolizumab is an anti-programmed death receptor-1 antibody that releases inhibition of the immune response, improving antitumor immunity [11,96].
Pembrolizumab is approved for any solid tumor with MSI-H or dMMR mutation that progresses during treatment without any satisfactory alternative treatment options [11,96]. This agent represented the first FDA approval (in 2017) with a biomarker-defined indication (i.e., agnostic of cancer site) [107]. Although this mutation is present in only about 1% of PDAC tumors, up to 83% of patients with dMMR PDAC respond to pembrolizumab [110].
Larotrectinib and Entrectinib
Larotrectinib and Entrectinib are neurotrophin receptor kinase (NTRK) inhibitors approved (in 2018 and 2019) for advanced, morbid, or unresectable solid tumors with NTRK fusion mutations, found in less than 1% of PDCAs [96].
The mutation product, TRK fusion protein, activates mitogen activated protein kinase-extracellular regulated kinase and phosphoinositide3 kinase-serine threonine signaling pathways, implicated in the oncogenesis of pancreatic cancer [96]. The NCCN recommends larotrectinib and entrectinib as first-line and subsequent treatment options for patients with NTRK gene fusion-positive locally advanced or metastatic PDAC [11].
A variety of data on chemotherapy efficacy are available, allowing for comparison of available agents in specific patient populations (Table 12). However, the terminology used can be confusing. Disease-free survival and progression-free survival are synonymous terms, and choice of the term used in this section will reflect the reference material. This is also the case with median survival and median overall survival. Unless noted otherwise, all patient outcomes are reported as median figures.
ADJUVANT CHEMOTHERAPY TRIALS IN RESECTABLE PDAC
| Phase III trial (Year) | Chemotherapy Comparison | Median Survival (Months) |
|---|---|---|
| ESPAC-1 (2004) | 5-FU vs. observation | 21 vs. 15.5 |
| CONKO-001 (2013) | Gemcitabine vs. observation | 22.8 vs. 20.2 |
| ESPAC-3 (2012) | Gemcitabine vs. 5-FU/leucovorin | 46 vs. 39 |
| ESPAC-4 (2017) | Gemcitabine/capecitabine vs. gemcitabine alone | 28 vs. 25.5 |
| PRODIGE 24 (2018) | Modified FOLFIRINOX vs. gemcitabine | 54.4 vs. 35 |
| APACT (2019) | Gemcitabine/paclitaxel vs. gemcitabine alone | 40.5 vs. 36.2 |
| 5-FU = 5-fluorouracil. | ||
The CONKO-001 trial established gemcitabine as standard adjuvant chemotherapy. In this study, 354 patients were randomized to receive gemcitabine or observation after resection and followed a median 136 months. Gemcitabine led to a 24% improvement in overall survival, a 10.3% absolute improvement in 5-year survival (20.7% vs. 10.4%), and a 4.5% improvement in 10-year survival (12.2% vs. 7.7%), compared to observation [111,112].
The ESPAC-3 trial showed the importance of completing the full post-resection adjuvant chemotherapy course (six cycles) in extending median overall survival of these patients compared with those not completing chemotherapy (28.0 months vs. 14.6 months) [96].
A continuation, ESPAC-4, found adding another fluoropyrimidine-based agent (capecitabine) to gemcitabine was superior to gemcitabine alone in median survival (28.0 months vs. 25.5 months) and five-year survival (28.8% vs 16.3%). A synergistic effect between gemcitabine and capecitabine on the DNA thymidylate enzyme was suggested [96].
PRODIGE-24 randomized 493 patients (ECOG performance status ≤1) with resected PDAC to modified FOLFIRINOX or gemcitabine for 24 weeks. At median 33.6 month follow-up, the disease-free survival with modified FOLFIRINOX was 21.6 months, compared with 12.8 months with gemcitabine [113]. Grade 3/4 toxicities were more frequent with mFOLFIRINOX (75.9%) than gemcitabine (52.9%). Nonetheless, the median 54.4-month overall survival with resection followed by mFOLFIRINOX is the longest survival reported to date with phase III results [5,114]. Thus, modified FOLFIRINOX is recommended as standard adjuvant chemotherapy in patients with excellent functional status; either gemcitabine/capecitabine or gemcitabine alone should be considered for individuals with poorer functional status [134].
Tolerance of adjuvant therapy remains a limitation, and patients commonly receive less than 50% of the planned dose, reflecting exposure to significant chemotherapy-related toxicity in patients experiencing substantial post-resection morbidity [2].
First-Line Chemotherapy in Metastatic PDAC
5-FU has been used in pancreatic cancer treatment since the 1950s. Patients with advanced PDAC typically show response rates greater than 20% and median survival of 2.5 to 6 months [24,80].
In 1997, gemcitabine replaced 5-FU as first-line treatment in metastatic PDAC by improving one-year survival rates (18% vs. 2%) and median overall survival (5.65 months vs. 4.41 months) [32]. Subsequently, numerous attempts to improve gemcitabine efficacy in metastatic PDAC have involved adding another cytotoxic drug [2,96]. Some show marginal but statistically significant improvements in median survival over gemcitabine alone (Table 13).
FIRST-LINE CHEMOTHERAPY TRIALS IN METASTATIC PDAC
| Phase III Trial (Year) | Chemotherapy Comparison | Median Survival (Months) | ||
|---|---|---|---|---|
| Cullinan (1985) |
| 5.5 vs. 5.5 vs. 4.5 | ||
| Burris (1997) | 5-FU vs. gemcitabine | 4.4 vs. 5.6 | ||
| Tempero (2003) | Gemcitabine vs. gemcitabine fixed dose rate | 5 vs. 8 | ||
| Heinemann (2006) | Gemcitabine ± cisplatin | 6.0 vs. 7.5 | ||
| NCIC-CTG PA.3 (2007) | Gemcitabine ± erlotinib | 5.9 vs. 6.2 | ||
| Cunningham (2009) | Gemcitabine ± capecitabine | 6.2 vs. 7.1 | ||
| CALGB 80303 (2010) | Gemcitabine ± bevacizumab | 5.9 vs. 5.8 | ||
| SWOG S0205 (2010) | Gemcitabine ± cetuximab | 5.9 vs. 6.3 | ||
| PRODIGE 4 (2011) | Gemcitabine vs. FOLFIRINOX | 6.8 vs. 11.1 | ||
| MPACT (2013) | Gemcitabine ± nab-paclitaxel | 6.7 vs. 8.5 | ||
| NAPOLI 3 (2023) | Gemcitabine + nab-paclitaxel vs. NALIRIFOX | 9.2 vs. 11.1 |
The NCIC CTG PA.3 trial found a nonmeaningful clinical improvement with gemcitabine/erlotinib over gemcitabine alone in median overall survival (6.24 months vs. 5.91 months). Despite FDA approval for locally advanced/metastatic PDAC, the clinical impact of this modest gain with increased toxicity can be questioned [32,96].
PRODIGE 4/ACCORD 11 demonstrated that patients with advanced PDAC and ECOG performance status ≤1 had better outcomes with FOLFIRINOX than gemcitabine in median overall survival (11.1 months vs. 6.8 months) and progression-free survival (6.4 months vs. 3.3 months). Following these findings, FOLFIRINOX became standard first-line therapy for candidate patients [2].
FOLFIRINOX was associated with more toxicities, but the six-month degradation in quality of life was better in FOLFIRINOX than gemcitabine (31% vs. 66%). Improved cancer control with FOLFIRINOX may be due to the inclusion of irinotecan, which has activity against PDAC and synergistic activity when given prior to 5-FU [96].
The MPACT study demonstrated an improvement of 1.8 months in both median overall survival and median progression-free survival with gemcitabine plus nab-paclitaxel versus gemcitabine alone, leading to another first-line option for metastatic PDAC [96].
In February 2024, the FDA approved irinotecan liposome with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX), for first-line treatment of metastatic pancreatic adenocarcinoma [140]. Efficacy was demonstrated in NAPOLI 3, a randomized, multicenter trial comparing NALIRIFOX with gemcitabine plus nab-paclitaxel in 770 patients previously untreated in the metastatic stage of disease. Treatment efficacy was superior in the NALIRIFOX arm for overall survival (11.1 months vs. 9.2 months) and for progression-free survival (7.4 months vs. 5.6 months) [140]. The most common adverse reactions of NALIRIFOX therapy (occurring n at least 20% of patients) were diarrhea, fatigue, nausea, vomiting, decreased appetite, abdominal discomfort, mucosal inflammation, and pancytopenia. NCCN guidelines now include NALIRIFOX in its list of preferred regimens for treatment of patients with metastatic pancreatic cancer [141].
The NAPOLI 3 trial results suggest that NALIRIFOX may have advantages over FOLFIRINOX for treatment of metastatic PDAC. The median progression-free survival was one month longer than that reported for FOLFIRINOX in the 2011 PRODEDGE 4 trial. A systematic review and analysis of seven clinical trials (2,581 patients) testing first-line therapy for metastatic pancreatic cancer found that therapeutic efficacy (progression-free and overall survival) was similar for NALIRIFOX and FOLFIRINOX, although both were superior to gemcitabine/nab-paclitaxel [142]. NALIRIFOX was associated with higher rates of severe diarrhea but less hematologic toxicity and lower incidence of peripheral neuropathy. No clinical trial directly comparing FOLFIRINOX to NALIRIFOX have been reported. The liposomal form of irinotecan in the NALIRIFOX regimen makes it considerably more expensive than FOLFIRINOX.
Second-Line Chemotherapy in Metastatic PDAC
Second-line therapy primarily consists of doublet therapy using the alternative pyrimidine backbone to what was used in the first-line setting. In 2016, the NAPOLI-1 trial demonstrated that after progression on a first-line gemcitabine-containing regimen for metastatic PDAC, 5-FU/leucovorin plus nanoliposomal irinotecan improved overall survival from 4.2 months (with 5-FU/leucovorin alone) to 6.1 months. As with nab-paclitaxel, improving the delivery of traditional chemotherapies may lead to more effective treatments for individuals with pancreatic cancer [32].
The POLO trial examined targeted maintenance therapy in a biomarker-selected population. In patients with metastatic PDAC harboring germline BRCA1/2 mutations who had not progressed on first-line platinum-based chemotherapy, those randomized to olaparib had improved median progression-free survival (7.4 months compared with 3.8 months with placebo), but olaparib did not improve median overall survival [109]. The median duration of response to olaparib was 6 months but was more than 24 months in a subset of patients (23%), which is exceptional in metastatic PDAC [108].
In second-line chemotherapy after progression on a first-line regimen, there is considerable heterogeneity in the survival of patients, and predicting which patients will benefit is not established. The decision to pursue second-line chemotherapy should be individualized and based on the patient's goals and preferences. Factors influencing the choice of second-line therapy include the regimen used for first-line therapy, performance status and comorbidity, and mutation status [106].
In addition to resection and chemotherapy, treatment of patients with PDAC may include radiation therapy or chemoradiotherapy. Unlike chemotherapy, the role of radiation therapy in the treatment of PDAC is uncertain. Radiation therapy is not a stand-alone treatment in local PDAC but is sequenced with chemotherapy as chemoradiotherapy.
Earlier adjuvant radiation therapy trials demonstrated an overall survival and disease-free survival benefit, but subsequent European chemoradiation studies showed negative findings [12]. Technical advances suggest increasing promise with radiation therapy, but multi-institutional randomized trials in PDAC have lagged [12].
Stereotactic body radiation therapy has promising local control and quality of life and is being evaluated for locally advanced and borderline resectable PDAC. However, adjuvant stereotactic body radiation therapy remains investigational with high toxicity risk and is only recommended as part of a clinical trial [12].
In the absence of phase 3 trials directly comparing neoadjuvant treatment approaches with or without radiation, adjuvant and neoadjuvant chemoradiation in PDAC awaits definitive evidence. Several such trials are in progress [2,12]. In particular, RTOG 0848 is expected to definitively clarify the role of post-resection radiotherapy [115].
Nonetheless, the prospective cohort and retrospective evidence suggestive of decreased local recurrence and disease progression is sufficient for ASTRO, the NCCN and ASCO to recommend radiation therapy. Standard radiation prescriptions in the neoadjuvant setting consist of daily treatments over the course of five or six weeks to a total dose of 50–54 gray (Gy) [2].
The type and duration of chemotherapy given with radiation therapy for pancreatic cancer depends on the clinical stage, setting (neoadjuvant or adjuvant), performance status, and comorbidities. Patients with favorable performance status (0 or 1) are typically offered FOLFIRINOX prior to radiation therapy. Patients who are elderly or have a poor performance status (≥2) are typically offered gemcitabine or gemcitabine/nab-paclitaxel prior to radiation therapy. The duration (two to six months or longer) depends on patient tolerance and tumor response (i.e., no evidence of progression on chemotherapy). Common dose-limiting toxicities are diarrhea, neuropathy, and hematologic [12].
Preoperative, or neoadjuvant, therapy is a major paradigm shift in treatment for patients with localized PDAC that offers the potential to lengthen survival while sparing patients unnecessary treatment-related morbidity using available treatments [116]. The purpose of neoadjuvant therapy differs somewhat in relation to disease stage and clinical features. Multiple prospective clinical trials have shown that neoadjuvant therapy eradicates occult metastatic disease, facilitates margin-negative surgical resection, and increases the number of patients eligible to receive postoperative adjutant chemotherapy [134].
Neoadjuvant therapy is recommended in upfront resectable disease with high-risk features of dissemination. This includes tumors in pancreas body and tail or >3–4 cm, ascites, large regional lymph nodes, CA19-9 levels >1,000 U/mL, severe weight loss, and extreme pain. For these patients, staging laparoscopy is recommended to identify liver and peritoneal metastases missed by MDCT in assessing resectability, with endoscopic ultrasonography-guided biopsy [7,11,15]. The next step is systemic neoadjuvant therapy (i.e., chemotherapy), post-neoadjuvant therapy CA19-9, and MDCT with contrast to reassess resectability (with some limitations). If R0 resection is feasible and there is no evidence of metastatic disease, surgery should be attempted [7,11,15].
In general, neoadjuvant therapy for patients who are candidates for resection is controversial [116]. Some oncology groups do not recommend neoadjuvant therapy in upfront resectable disease (except with high-risk features) until better evidence is available, but this stance has become less tenable as additional evidence supporting efficacy becomes available [7,13,15].
Even in patients with anatomically localized disease based on imaging and after complete resection with R0 margins, the high rates of distant failure after surgery for resectable PDAC indicates most patients already have systemic disease at the time of diagnosis. Current imaging fails to accurately assess the true burden of disease, missing occult metastases and under-staging patients [116].
Given this reality, systemic therapy is crucial, but many patients do not receive adjuvant therapy after resection. The high complication rates and potentially prolonged recovery with resection results in 25% to 50% of patients not receiving postoperative therapy [116]. However, systemic neoadjuvant therapy allows patients to receive therapy when they have better performance status and before the potential development of postoperative complications [116].
Neoadjuvant therapy also tests the tumor biology. Patients with aggressive tumors that progress and/or metastasize during neoadjuvant therapy are spared a futile operation. Due to their performance status, patients who do poorly on systemic neoadjuvant therapy would likely do poorly with surgery, resulting in mortality or serious perioperative morbidity precluding adjuvant therapy. Neoadjuvant therapy allows patients with resectable tumors who are poor surgical candidates time to medically and/or physically optimize before surgery.
Neoadjuvant therapy is not without its drawbacks. Eligibility for neoadjuvant therapy requires a tissue diagnosis, but the dense PDAC tumor stroma impedes tissue confirmation in approximately 15% of patients [116]. Further, neoadjuvant therapy means delaying surgery, with the possibility for local progression during neoadjuvant therapy into unresectable PDAC [15]. However, local progression almost always occurs concomitantly with development of systemic disease [116]. Essentially, better evidence is needed. Until phase III results are available, the poor outcomes of conventional treatment sequencing argue for the need for neoadjuvant therapy.
Borderline resectable pancreatic cancer is a recognized indication for neoadjuvant therapy, as this approach may shrink and make tumors more amenable for surgical resection with fewer complications and increased chance of R0 resection. Neoadjuvant therapy may minimize early non-detectable microscopic metastases, decrease lymph node involvement, and improve overall survival and outcomes [96].
The NCCN recommends neoadjuvant therapy for patients with resectable or borderline resectable tumors. Treatment at or coordinated through a high-volume center is preferred, when feasible, and participation in a clinical trial is encouraged. The preferred neoadjuvant options are FOLFIRINOX with or without subsequent chemoradiation, or gemcitabine plus albumin-bound paclitaxel with or without subsequent chemoradiation [11]. For patients with BRCA/PALB2 mutations, the preferred regimen is gemcitabine plus cisplatin (two to six cycles) with or without subsequent chemoradiation [11].
ASTRO guidelines for neoadjuvant chemoradiation specify a radiation dose of 4,500–5,040 cGy in 180–200 cGy fractions [12]. They recommend delivery of radiation therapy following two to six months of chemotherapy.
Locally advanced pancreatic cancer accounts for 30% of newly diagnosed cases. With local involvement of adjacent critical blood vessels and presence of occult micrometastatic disease, locally advanced pancreatic cancer is generally considered surgically unresectable and incurable, and the standard of care is as for metastatic disease [2].
However, the increased use of preoperative multiagent chemotherapy followed by chemoradiation has significantly expanded the pool of patients with locally advanced pancreatic cancer eligible for resection with curative intent, significantly improving the resectability and overall survival of these patients [117].
In a single-institution phase II trial, 49 patients with locally advanced pancreatic cancer received eight cycles of FOLFIRINOX followed by 50.4 Gy of photon radiation with capecitabine and losartan. Of these patients, 39 were brought to the operating room, 34 (69%) had their cancer removed, and of these, 30 patients (88%) had an R0 resection. Among patients who underwent resection, median progression-free survival and overall survival were 21.3 and 33 months, respectively, versus the 11- to 12-month historical overall survival [118].
Neoadjuvant therapy is associated with a downstaging-to-resection rate of greater than 30% in selected patients with locally advanced pancreatic cancer, with survival comparable to or better than initially resectable disease. For patients with arterial involvement, arterial divestment shows a lower morbidity and mortality rate than arterial resection and reconstruction [117].
Following neoadjuvant therapy, a restaging evaluation with pancreatic protocol MDCT is required to image tumor shrinkage and rule out local progression for resectability. However, post-neoadjuvant therapy imaging is not a reliable indicator of resectability due to its inability to distinguish post-treatment fibrosis from residual viable tumor [117]. Post-neoadjuvant therapy CA19-9 levels are predictive of tumor regression and should be used to guide decisions about suitability for surgical exploration for resection. Diagnostic laparoscopy should be routinely used to minimize nontherapeutic surgery rates [117].
After resection of pancreatic cancer following neoadjuvant FOLFIRINOX, the benefit of adjuvant chemotherapy on overall survival is unclear. Although randomized controlled trial confirmation is needed, a 2020 multicenter, retrospective study provided informative results [119]. Of 520 patients (median age: 61 years; 53.7% male) who received a median of six neoadjuvant cycles of FOLFIRINOX, 343 (66.0%) received adjuvant chemotherapy. Adjuvant chemotherapy was FOLFIRINOX for 68 patients (19.8%), gemcitabine-based chemotherapy for 201 (58.6%), capecitabine for 14 (4.1%), a combination or other agents for 45 (13.1%), and unknown for 15 patients (4.4%). The median overall survival was 38 months after diagnosis and 31 months after surgery. No survival difference was found for patients who received adjuvant chemotherapy compared with those who did not (29 months in both groups).
In multivariable analysis, the interaction of lymph node stage with adjuvant therapy was statistically significant. In patients with pathology-proven node-positive disease, adjuvant chemotherapy was associated with improved overall survival (26 months vs. 13 months). For those with node-negative disease, adjuvant chemotherapy was not associated with improved survival (38 months vs. 54 months). These results suggest that adjuvant chemotherapy after neoadjuvant therapy FOLFIRINOX and resection of pancreatic cancer was associated with improved survival only in patients with pathology-proven node-positive disease [119].
Neoadjuvant therapy increasingly shows the ability to downstage locally advanced pancreatic cancer into resectable tumor, but until such approaches are employed beyond specialized PDAC research centers, most of these patients will remain unresectable [2].
Chemotherapy selection for patients with locally advanced pancreatic cancer is largely based on extrapolation from studies in metastatic PDAC. However, the natural history of locally advanced pancreatic cancer is less predictable than metastatic disease [120]. In an important autopsy study, 28% of patients with locally advanced pancreatic cancer at initial diagnosis died with localized disease only, from complications of locally destructive tumor growth [120]. Also noted, not all isolated metastases at initial diagnosis are harbingers of widespread metastatic disease, nor the greatest threat to patient survival compared with the primary tumor or cachexia [17].
In patients with locally advanced pancreatic cancer, even with progression, treatment should not simply mirror that in metastatic disease. Rather, it should be based on the pattern of progression (locoregional vs. disseminated), prior chemotherapy and/or radiation, and sequence of therapy (as well as performance status and comorbidity). For example, if a patient with locally advanced pancreatic cancer and a history of only chemotherapy as prior treatment later develops locoregional progression, radiation may be the appropriate modality [8].
Fluoropyrimidines and gemcitabine are the most used agents in adjuvant chemoradiotherapy trials of locally advanced pancreatic cancer. These studies suggest that as a radiosensitizer, capecitabine is a well-tolerated regimen with comparable or superior outcomes compared with low-dose gemcitabine [8].
There is a potential role for maintenance capecitabine or gemcitabine-based chemoradiotherapy in improving quality of life for patients with locally advanced pancreatic cancer and stable disease after 12 weeks of induction gemcitabine/capecitabine chemotherapy [8].
In contrast to conventionally fractionated chemoradiotherapy, there is growing interest in using induction chemotherapy for systemic control, followed by a short course of stereotactic body radiotherapy early during treatment with minimum disruption to systemic therapy. This could be particularly beneficial to patients with predominant local symptoms [8].
The ASCO guidelines for patients with locally advanced pancreatic cancer include several strong recommendations related to chemoradiotherapy or stereotactic body radiation therapy [2,8]. Specifically, it states that chemoradiotherapy or stereotactic body radiation therapy may be offered upfront rather than chemotherapy [8]. This approach is recommended for patients with local progression but no metastases, performance status ≤2, and favorable comorbid profile. It should also be offered to patients with response to an initial six months of chemotherapy or with stable disease who develop chemotherapy toxicities that are intolerable or cause a decline in performance status [8]. If patients respond or their disease has at least stabilized after six months of induction chemotherapy, chemoradiotherapy or stereotactic body radiation therapy may be offered as an alternative to continuing chemotherapy alone [8].
For patients with unresectable or locally advanced pancreatic cancer, definitive conventionally fractionated or dose-escalated radiation therapy with chemotherapy is used. For patients without systemic progression after four to six months (or longer) of chemotherapy, ASTRO recommends definitive radiation therapy [12]. The preferred dose is 5,040–5,600 cGy in 175–220 cGy fractions.
With surgical resection considered the only potentially curative option but most patients harboring unresectable PDAC tumor, nonoperative local treatment options that can provide a similar benefit are needed. Emerging radiation techniques that address organ motion have enabled curative radiation doses delivered in patients with inoperable disease [121].
In one 2021 report, patients with locally advanced pancreatic cancer were treated with hypofractionated ablative radiation therapy, using respiratory gating, soft tissue image guidance, and other methods to address organ motion and limit the dose to surrounding luminal organs [121]. At baseline, 119 patients with locally advanced pancreatic cancer and median CA19-9 level >167 U/mL received four months of induction chemotherapy, followed by ablative radiation therapy. The median overall survival from diagnosis and ablative radiation therapy were 26.8 and 18.4 months. The 12- and 24-month overall survival following therapy were 74% and 38%, and the 12- and 24-month cumulative incidence of locoregional failure were 17.6% and 32.8% [121]. Postinduction CA19-9 decline was associated with improved locoregional control and survival. Grade 3 upper GI bleeding occurred in 10 patients (8%), with no grade 4 to 5 events. This cohort study of patients with inoperable locally advanced pancreatic cancer found that ablative radiation therapy following multiagent induction therapy was associated with durable locoregional tumor control and favorable survival [121].
Systemic chemotherapy can benefit patients with metastatic PDAC by improving disease-related symptoms and survival compared with best supportive care alone, but patients should understand that chemotherapy is palliative and not curative [80].
First-line chemotherapy for metastatic PDAC is consistent across clinical practice guidelines from ASCO, NCCN and ESMO. The preferred regimens are gemcitabine/albumin-bound paclitaxel, FOLFIRINOX, mFOLFIRINOX, and NALIRIFOX. Treatment selection is based on PDAC mutation status, serum total bilirubin level, ECOG performance status, comorbidity profile, patient preference and a support system for aggressive medical therapy, and access to chemotherapy port and infusion pump management services.
The initial chemotherapy selection for germline or somatic HRR gene mutation is a platinum-based chemotherapy regimen. For those with performance status ≤1 and serum bilirubin less than 1.5 times upper limit of normal, FOLFIRINOX or mFOLFIRINOX is preferred. Gemcitabine plus cisplatin can be used and probably has similar benefit. For patients with performance status 2, comorbidity that precludes intensive therapy, or a serum bilirubin more than 1.5 times upper limit of normal despite stenting, FOLFOX is preferred over FOLFIRINOX.
After at least 16 weeks of initial platinum-based chemotherapy without disease progression, chemotherapy should be discontinued and maintenance therapy with olaparib initiated for those with germline BRCA or PALB2 mutation. For advanced PDAC with somatic (i.e., non-germline) BRCA or PALB2 mutation, the benefit of olaparib maintenance therapy is not known and is under investigation.
For patients with an unknown (pending) HRR status, waiting until the germline or somatic mutation status is known is not recommend, given the rapidity of progression in most patients with newly diagnosed metastatic PDAC. These patients should be treated like HRR mutation carriers until results of genetic testing are available [80].
Patients with performance status ≤1, serum bilirubin less than 1.5 times upper limit of normal, and favorable comorbidity, FOLFIRINOX or NALIRIFOX is preferred, with gemcitabine plus nabpaclitaxel a potentially less toxic alternative. Patients with serum bilirubin more than 1.5 times upper limit of normal despite placement of a stent should receive FOLFOX rather than a gemcitabine-containing regimen, because gemcitabine is hepatically metabolized and associated with greater toxicity with hepatic impairment. For patients with performance status 2, favorable/adequate comorbidity, and serum bilirubin level less than 1.5 times upper limit of normal, gemcitabine monotherapy is suggested; gemcitabine/capecitabine is another option.
Highly selected patients with performance status 2 due to heavy tumor burden should be treated with gemcitabine plus nabpaclitaxel, owing to its higher response rate. Dose and schedule adjustments should be made to minimize toxicities. In patients with performance status ≥3 or poorly controlled comorbidity (regardless of histology or BRCA/PALB2 mutation status), systemic chemotherapy should only be offered on an individualized, case-by-case basis; supportive care should be emphasized.
At diagnosis, the median survival for patients with locally advanced, unresectable pancreatic cancer is 8 to 12 months; with metastatic disease, this decreases to 3 to 6 months. For patients with locally advanced and metastatic disease, systemic chemotherapy can improve survival. In the best outcomes to date, FOLFIRINOX demonstrated an 11.1-month median survival [122].
Patients receiving chemotherapy often report better overall quality of life, but extended survival with chemotherapy may not reduce symptom burden. Because the pancreas is located deep in the central abdomen at the root of the mesentery and adjacent to the biliary and gastrointestinal tracts, most patients suffer a varied and serious symptom burden, frequently requiring medical attention and hospitalization for effective management. The common symptoms experienced by patients with PDAC are pain, abdominal distension/bloating, anorexia, constipation, anxiety, and depression. Diarrhea and steatorrhea are also common. Most patients experience significant weight loss and become cachectic, which further reduces quality of life, treatment response, and length of survival. Other intercurrent complications include biliary obstruction, gastric outlet obstruction, ascites, and venous thromboembolism [122,143].
All patients with newly diagnosed PDAC should have a full assessment of symptom burden, psychological status, and social supports as early as possible. Regardless of cancer stage and patient prognosis, early introduction to expert palliative and supportive care improves the social, psychological, and physical well-being of patients; decreases the intensity of medical interventions at the end of life; and ultimately improves survival [2].
Palliative care is an interdisciplinary specialty that is focused on preventing and relieving suffering and supporting the best possible quality of life for patients and their families facing serious illness, such as pancreatic cancer. Palliative care specialist clinicians provide in-depth pain and symptom management, communication regarding goals of care, and coordinated care across settings and over time. Palliative care aims to relieve suffering in all stages of disease and can be provided in tandem with curative or life-prolonging treatments [122].
When initiated early in the disease course, palliative care improves clinical, quality of care, and survival outcomes. Furthermore, multiple studies have shown that palliative care services improve patients' symptoms, allow patients to avoid hospitalization and to remain safely and adequately cared for at home, lead to better patient and family satisfaction, and significantly reduce prolonged grief and post-traumatic stress disorder among bereaved family members. Palliative care also lowers costs and reduces rates of unnecessary hospitalizations, diagnostic and treatment interventions, and nonbeneficial intensive care when patients are near the end of life [122].
Acquired hypercoagulability is a common feature of pancreatic cancer, which is one of the highest-risk malignancies for venous thromboembolism (VTE), including deep venous thrombosis (DVT), pulmonary embolism, and visceral portal or superior mesenteric vein thrombi. The reported incidence of VTE in patients with advanced PDAC is greater than 25%, which is four- to seven-fold higher than any other malignancy and fifty times higher than the average adult [143]. The risk is highest in the first three months after diagnosis; chemotherapy further increases the risk. In PDAC, VTE is strongly associated with higher short- and long-term mortality and high risk of recurrent VTE [122].
All patients should be educated about warning signs and symptoms of VTE. Physical examination of the legs for asymmetric pitting edema, erythema, and warmth is crucial in each office visit, and the threshold to perform a CT angiogram with tachycardia or pleuritic chest pain present should be extremely low [122].
Routine anticoagulation for primary VTE prevention is not indicated in ambulatory outpatients with pancreatic cancer and no other VTE risk factors [122]. In a patient with PDAC and documented VTE (symptomatic or incidentally found), early initiation of anticoagulation is the standard approach, and lifelong therapy should be considered. The decision to continue anticoagulation should be balanced against bleeding risk, cost of therapy, quality of life, life expectancy, and patient preference. Low-molecular-weight heparin or oral rivaroxaban, apixaban, or edoxaban is preferred to vitamin K antagonist or unfractionated heparin for long-term anticoagulation [122].
Symptomatic biliary obstruction develops in approximately 80% of patients with carcinoma of the head of the pancreas [143]. Biliary stents relieve troublesome pruritis and may be used to improve liver function sufficiently to permit safe administration of cancer treatment. Endoscopic retrograde stenting is superior to surgical or percutaneous approaches to address bile duct obstruction because of a more favorable adverse event rate. Successful biliary stent placement can be achieved in more than 90% of patients, with potential post-procedure complications of pancreatitis, bleeding, or cholangitis in 5% of cases [143]. Self-expandable metal stents are preferred over plastic stents in patients with a life expectancy of more than three months in terms of patency duration, less therapeutic failure and need for reintervention, lower cholangitis incidence, and better patient quality of life. Patency rates between covered and uncovered metal stents are not significantly different [16]. Endoscopic ultrasonography-guided biliary drainage is an alternative if endoscopic biliary stent placement is unsuccessful or technically not feasible.
Pancreatic cancer invasion into the duodenum can lead to secondary gastric outlet obstruction and intractable nausea and vomiting. The choice of treatment intervention depends on the functional status and patient's predicted length of survival [143]. In patients with gastric outlet obstruction, endoscopic duodenal stenting allows a quick resumption of oral intake, with a low complication rate and a short recovery period. However, the need for reintervention is higher after duodenal stenting compared with that of palliative surgery. Endoscopic ultrasonography-guided gastrojejunostomy is an effective and safe alternative to surgery [16].
Ascites in patients with metastatic PDAC may be due to peritoneal metastases. In patients with locally advanced tumors, ascites may be caused by portal vein thrombus if the tumor compresses the portal vein locally [122].
Patients with malignant ascites from pancreatic cancer can experience abdominal discomfort, nausea, vomiting, and dyspnea from the pressure of the fluid against the anterior abdominal wall and diaphragm. For most patients, survival is short, and the focus is symptom control. Symptom relief from intermittent paracentesis tends to be short-lived, and the procedure must be repeated for symptom relief. If reaccumulation requires more than once-weekly paracentesis, placement of a long-term drainage catheter is an option; complication rates are higher with indwelling catheters. Diuretics such as spironolactone and furosemide decrease the absorption of water and sodium in the kidneys and may provide some symptomatic relief [122].
Pancreatic cancer is one of the most painful malignancies [85]. All patients with locally advanced and metastatic pancreatic cancer should be offered aggressive treatment of pain [8]. Adequate control of pain may be unsatisfactory due to significant variation in local practice [123].
Abdominal and/or back pain is often the major presenting symptom of the disease and can be a significant feature of advanced pancreatic cancer. Patients describe a gnawing mid-epigastric pain, which radiates bilaterally under the ribs and into the mid-back, owing to the proximity of pancreatic tumors to the celiac plexus. All patients should have the level of pain and degree of pain relief from analgesics addressed at every visit [122].
The mainstay of pain management is opioid therapy, and palliation of pain can often be successfully achieved by opioid analgesics alone [122]. The core principle is a "pain/analgesia ladder," with escalation in management based on symptom severity. Initial treatment may consist of non-opioid medications (e.g., non-steroidal anti-inflammatory drugs [NSAIDs]), followed by mild/moderate opioids (e.g., tramadol, codeine), then stronger opioids (e.g., morphine, oxycodone, fentanyl) for unremitting and severe pain. At each level of pain management, adjunctive therapy (e.g., cannabinoid, ketamine, clonidine, benzodiazepine, gabapentin, pregabalin, nortriptyline, duloxetine) can be incorporated for potential additive effect [122,143].
Patients with moderate-to-severe pain should receive doses adequate to provide relief. Concern about addiction should not be a barrier to effective pain control; even with dose escalation, addiction is seldom a problem in patients with PDAC and the risk is lower than generally assumed in non-malignant pain [81,123]. Given the ongoing concerns regarding opioid misuse in the United States, drug diversion may be a consideration.
For patients with persistent nausea and vomiting for whom taking oral medications is difficult, pain control may be achieved using transdermal patches when adipose tissue is sufficient for transdermal absorption [122]. When pain is constant rather than intermittent, long-acting oral (e.g., morphine, oxycodone, oxymorphone) or transdermal (e.g., fentanyl, buprenorphine) preparations may work better [81]. Breakthrough pain can be treated with rapid-onset transmucosal or intranasal fentanyl formulations. Methadone may be advantageous in many patients and can be used in small doses as add-on to existing opioid treatment. Methadone should only by prescribed by clinicians who are familiar with the complex pharmacology and adverse effect profile of this opioid [123].
Laxatives should be considered for all patients on opioid analgesia for PDAC pain, because constipation is a nearly universal side effect. There is considerable individual variation in both efficacy and side effects. Not all patients benefit from or tolerate opioids. A trial of an alternative opioid may also be indicated. Cases of poor pain control or intolerable pain may benefit from continuous opioid infusion via epidural or intrathecal catheters [81,123].
Near the end of life, pain management for advanced and terminal PDAC can become very challenging, and an interdisciplinary approach including palliative care specialists is needed. It is important wherever possible to consider the preferences of the patient. A range of supportive care measures can be offered, including intensive home support, home care with parenteral opioids, patient-controlled analgesia, and palliative sedation [123].
Celiac plexus neurolysis offers medium-term relief, but other procedures (e.g., splanchnicectomy) are also available. Adjunctive treatments for pain, depression, and anxiety as well as radiotherapy, endoscopic therapy, and neuromodulation may be required. Palliative chemotherapy may provide pain relief as a collateral benefit [123].
Interventional therapies may be beneficial for severe pain refractory to opioids. A celiac plexus block uses a local injection of corticosteroids or a long-acting analgesic to reduce pain by disrupting visceral pain afferent pathways from the pancreas and surrounding structures. A celiac plexus neurolysis is permanent destruction of the plexus, equally effective and preferable for patients with a short life expectancy as it may provide symptomatic relief for three to six months [143].
The celiac plexus is a dense network of nerves that innervates the upper abdominal organs. Pain may be relieved by inhibiting synaptic pathways within the plexus by chemical destruction of the pathways and ganglia using dehydrated alcohol. Celiac plexus neurolysis is performed under endoscopic ultrasonography guidance [122].
Celiac plexus neurolysis improves analgesia and quality of life and decreases opioid requirements. The analgesic effect seems to vanish after eight weeks, and in most patients, pain recurs after three months. Repeated celiac plexus neurolysis benefits about 30% of patients and is normally not offered [123].
Splanchnicectomy may disrupt more nerve pathways than celiac plexus neurolysis and is a better option when there is a large mass in the region of the celiac plexus. Splanchnicectomy is seldom performed in patients with PDAC despite some evidence of long-lasting pain relief and few complications in observational series, possibly because the expertise is not widely available [123].
External beam radiation therapy with or without concomitant chemotherapy may also significantly alleviate pain due to local invasion of pancreatic cancer, frequently with improvement in cachexia and obstructive symptoms. However, it may take several weeks to achieve its maximal effect. When pain is caused by liver or bone metastases, patients may benefit from radiation therapy [16,122].
Nutritional compromise in PDAC is common, but the underlying pathologies are diverse [2]. Nausea, caused both by the primary disease process and its associated chemotherapy, is most effectively treated with serotonin-3 receptor antagonists and atypical antipsychotics (e.g., olanzapine), with some emerging evidence suggesting efficacy with cannabinoids. Loss of appetite, even in the absence of overt nausea, is frequently reported by patients, and this symptom is driven by central pathways that are largely distinct from those that produce nausea.
Malabsorption secondary to pancreatic exocrine deficiency degrades nutritional status. Pancreatic enzyme-replacement therapy helps to stabilize weight loss and also improves quality of life by decreasing gastrointestinal symptoms. Malabsorption from biliary obstruction is a complication found in up to 90% of patients with PDAC. Similar to the replacement of pancreatic enzymes, the treatment of biliary obstruction improves symptoms beyond its effects on digestion, including anorexia, pruritus, and fatigue.
Collectively, careful attention to the nutritional status of patients with PDAC improves both their survival and quality of life. Early and regular involvement of nutrition experts in their care is recommended [2,124].
A constellation of disproportionate loss of lean body mass, weight loss, muscle wasting, adipose tissue reprogramming, and anorexia, cancer-related anorexia/cachexia syndrome (CACS) is more frequent in patients with PDAC than in any other malignancy due to the complex metabolic profile of pancreatic cancer [2]. In a study of 390 patients with advanced cancers, the rate of cachexia was highest in PDAC (89%), followed by gastric cancer (76%) and esophageal cancer (53%) [125].
Unlike simple starvation, which is characterized by a caloric deficiency that can be reversed with appropriate feeding, the weight loss of cachexia cannot be adequately treated with aggressive feeding [126]. The physical impact of CACS contributes to decreased patient quality of life, treatment response, and survival due to gross alterations in protein metabolism, increased oxidative stress, and systemic inflammation. The psychological impact also contributes to decreased quality of life for both patients and their families [125].
In CACS, an abnormally accelerated resting energy expenditure increases muscle protein breakdown and lipolysis, which seems related to activation of cytokines (e.g., tumor necrosis factor-alpha, interleukin 6 and 1 beta), and tumor-derived, potentially cachexia-inducing factors that target skeletal muscle gene products [122,126].
Potentially Beneficial Agents
Cachexia does not respond to nutritional support. There are no FDA-approved medications for treatment of CACS, and positive pharmacotherapy response in patients with anorexia associated with non-malignant disease has been difficult to translate into benefit for patients with cancer [127,128].
Many agents have been evaluated for the treatment of CACS, but only corticosteroids (e.g., dexamethasone) and progesterone analogs (e.g., megestrol acetate) have a proven benefit in the anorexia associated with this syndrome [122]. Selection is based on life expectancy and assessment of risks versus benefits. Dexamethasone is suggested for patients for whom only weeks of therapy are anticipated, while megestrol acetate or medroxyprogesterone acetate (another progesterone analog) are suggested for patients with longer life expectancies [126].
A phase III study randomized 190 patients with advanced cancer and anorexia to megestrol acetate (480 mg/day), dexamethasone (4 mg/day), or placebo for up to four weeks. Differences in primary endpoint (at least 25% improvement in appetite) between megestrol (79.3%), dexamethasone (65.5%), and placebo (58.5%) were non-significant. Hyperglycemia and deep vein thromboses were more frequent with dexamethasone than megestrol or placebo. No other differences from placebo were found [127].
In this trial, the higher rate of deep vein thromboses with dexamethasone was unexpected. Megestrol acetate is associated with thromboembolic events and is contraindicated in patients with VTE. Dexamethasone has the potential to reduce cancer-related fatigue and elevate mood, at the significant cost of accelerating catabolic effects on muscle [127]. The primary benefits associated with these drugs are increased appetite and weight gain, not improved survival, and both drugs are associated with potential harms [122].
Mirtazapine is well-known for promoting weight gain. A placebo-controlled randomized trial found that appetite scores increased similarly with mirtazapine (15 mg at night) and placebo during the 28-day study. Mirtazapine was associated with significantly less increase in depressive symptoms and higher prevalence of somnolence than placebo, but no other differences were found [128].
The evidence of benefit in patients with CACS is inconclusive for androgens and selective androgen receptor modulators, anamorelin, cyproheptadine, long-chain omega-3 fatty acids, vitamins, minerals, and other dietary supplements, nonsteroidal anti-inflammatory drugs (NSAIDs), thalidomide, and combination approaches [126]. However, a trial of low-dose olanzapine (5 mg/day) is reasonable, particularly for patients who have concurrent nausea and/or vomiting unrelated to chemotherapy or radiation therapy [126].
Cannabis and Cannabinoids
In the cannabis plant, delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best-characterized therapeutic constituents. Pharmaceutical cannabinoid products containing THC (dronabinol), a THC analog (nabilone), or THC:CBD in an oromucosal spray (nabiximols, investigational) were examined for efficacy in CACS and palliative care in two meta-analyses [126].
Unfortunately, no benefit beyond placebo was found for pharmaceutical cannabinoid products in CACS, despite their superior weight gain and appetite effects in patients with advanced HIV [129]. Cancer patients with more than 30% decrease in pain with cannabinoids compared with placebo approached significance [129].
In both meta-analyses, available studies of smoked cannabis in CACS did not meet evidence thresholds and were excluded. This limits the ability to inform real-world clinical practice, where patient preference, self-titration to tolerability/effect, access, and other factors favor smoked/vaped cannabis over single-molecule pharmaceutical cannabinoids [130].
Counseling and Support
The substantial loss of body mass can cause significant distress to patients. Although advanced cachexia is irreversible, palliating anorexia in patients with advanced cancer is best approached by focusing on stimulating appetite, supporting each person's food preferences, and avoiding prescriptive dietary advice [127].
Providing education to patients and their caregivers is crucial. The objective is to promote a shared understanding about changed goals of care, and to help reduce the distress caused by reduced oral intake [127].
Counselling of family members regarding what to expect with disease progression is important—it can alleviate distress that leads to well-meaning but futile attempts to pressure or coerce the patient into increased feeding. Key points to discuss with patients and their family members, related to interactions about nutrition and eating near the end of life, include the following [131]:
Loss of appetite is common in patients with advanced cancer and may be the result of the cancer process itself.
Trying to force a patient to eat is usually counterproductive, potentially leading to increased nausea/vomiting.
In most patients with advanced cancer and cachexia, providing additional calories by feeding tubes and/or intravenously does not improve outcomes.
Trying to make a patient eat, when they have marked appetite loss, can lead to decreased social interactions and increased patient distress regarding interactions with caregivers (including stories of patients, in their dying days, pretending to be asleep when relatives visit, so that the relatives do not try to make them eat something).
Caregivers should be advised that it may be best to listen to and support the patient in a variety of other ways (such as giving the patient a massage or applying a lip moisturizer) instead of trying to talk them into eating more. Referral to a registered dietitian may provide patients and caregivers with additional opportunities to discuss concerns and challenges related to nutrition, appetite, and meal planning.
The presence of diabetes has been associated with higher mortality in patients with PDAC; corticosteroids can induce or exacerbate diabetes in these patients. For patients with PDAC-related diabetes, nutritional management by an experienced dietitian is essential [16]. Metformin or insulin is used as a first-line therapy. Insulin is often the preferred agent because of its efficacy, flexibility, and safety.
Careful monitoring of plasma glucose levels two hours after meals is widely recommended. The limited literature on this topic recommends maintaining blood glucose levels to avoid hypoglycemia and reduce symptoms of hyperglycemia.
A contributory factor to extreme weight loss may be pancreatic exocrine insufficiency, which leads to maldigestion, fat malabsorption, and steatorrhea. This complication may be caused by gradual tumor encroachment on pancreatic parenchyma or obstruction of the main pancreatic duct, or as a complication of surgery and/or irradiation. The main clinical manifestation is weight loss and malnutrition, and nonspecific symptoms such as abdominal cramping, flatulence, and urgency to defecate. Fat malabsorption does not become evident until pancreatic lipase secretion falls below 10% of normal levels [122]. The characteristic fatty stools associated with steatorrhea (loose, greasy, foul-smelling) may not be evident because patients tend to limit fat ingestion.
Pancreatic exocrine insufficiency is very frequent (>90% with tumors in the pancreatic head), and is associated with higher mortality in patients with unresectable PDAC. Pancreatic enzyme replacement therapy (PERT) improves survival in these patients [16]. Given its high incidence, diagnostic testing is not necessary. Patients suspected of fat malabsorption should be treated empirically with oral PERT [122].
The classical approach to patients with pancreatic exocrine insufficiency was restricting fat intake (<20 gm/day) to reduce steatorrhea. However, this further restricts the intake of fat-soluble vitamins, which are already malabsorbed in patients with pancreatic exocrine insufficiency and is not recommended. Frequent low-volume meals and avoidance of foods that are difficult to digest (e.g., legumes) are generally recommended [122].
Pancreatic exocrine insufficiency is treated with capsules of porcine pancreatic enzymes (pancrelipase). There are several commercial products available, and the amount of enzyme per capsule varies [81]. Doses are in United States Pharmacopeia (USP) units or International Units (IU); 90,000 USP is equivalent to 30,000 IU [122]. A healthy pancreas produces about 900,000 USP of lipase in response to a meal. Sufficient fat absorption can be maintained at around 10% of normal capacity; thus, roughly 90,000 USP per meal is needed. Because non-resected patients retain some pancreatic function, a starting dose of 75,000 USP with main meals and 25,000 with snacks should suffice in reducing steatorrhea and preventing weight loss. Enzymes are most effective when taken across the course of a meal. Following Whipple, patients will require 90,000 USP with meals and 45,000 USP with snacks [124].
Acidic gastric pH is normally neutralized by pancreatic bicarbonate secretion, which is absent in many patients with PDAC, especially following Whipple resection. Acid-suppressing therapy with a proton pump inhibitor is often required, as failure to neutralize gastric acid inactivates the enzymes [16,124].
Despite recommendation from expert groups, including the NCCN, evidence suggests PERT is underutilized. This was examined in a large commercially insured U.S. population from 2001–2013. Among patients with PDAC (32,461), 1.9% had diagnostic testing for exocrine insufficiency, 21.9% filled a prescription for PERT, and 5.5% were prescribed an adequate dose (defined as ≥120,000 USP lipase daily) [132].
Testing and appropriate dosing is infrequent and inconsistent in an insured U.S. population. Efforts are needed to educate medical providers on the best practices for managing exocrine pancreatic insufficiency in these patients [132].
For patients who are not proficient in English, it is important that information regarding all aspects of their care (including diagnostic procedures and treatment options) and palliative care resources be provided in their native language, if possible. When there is an obvious disconnect in the communication process between the practitioner and patient due to the patient's lack of proficiency in the English language, an interpreter is required. Interpreters can be a valuable resource to help bridge the communication and cultural gap between patients and practitioners. Interpreters are more than passive agents who translate and transmit information back and forth from party to party. When they are enlisted and treated as part of the interdisciplinary clinical team, they serve as cultural brokers who ultimately enhance the clinical encounter. In any case in which information regarding treatment options and medication/treatment measures are being provided, the use of an interpreter should be considered. Print materials are also available in many languages, and these should be offered whenever necessary.
PDAC is the most lethal solid malignancy, predicted to become the second leading cause of cancer death in the United States by 2030. The complexity of this aggressive cancer has been vexing to investigators and tragic for patients and their families. Major research efforts over the past 50 years have improved the five-year survival rate from 6% to 12.8%. The greatest gains—from resection of early-stage tumors—are the least likely to present at diagnosis. There is an urgent need to reduce PDAC incidence through primary and secondary prevention, and mortality by accelerating therapeutic development [133].
Until diagnostic or therapeutics breakthroughs arrive, novel uses of standard treatments (i.e., neoadjuvant therapy) show survival advantages for a greater number of patients. The longest survival reported by a phase III trial was published in 2018—a median 54.4 months in patients who received resection followed by mFOLFIRINOX [113]. Many novel treatments are in phase III trials. Additional approaches to manage morbidities and provide better palliative care are also needed. Cancer anorexia/cachexia is a high-priority area.
It is now clear that even early-stage PDAC is a systemic disease and that new-onset metabolic (e.g., diabetes, anorexia/cachexia, hyperglycemia) and neuropsychiatric (e.g., depression, fatigue) symptoms/syndromes are prodromal rather than comorbid or secondary. This recognition has also called for a re-thinking of pancreatic cancer from a more integrative, multi-system perspective [2].
1. Hall BR, Cannon A, Atri P, et al. Advanced pancreatic cancer: a meta-analysis of clinical trials over thirty years. Oncotarget. 2018;9:19396-19405.
2. Grossberg AJ, Chu LC, Deig CR, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. 2020;70:375-403.
3. Lei F, Xi X, Batra SK, et al. Combination therapies and drug delivery platforms in combating pancreatic cancer. J Pharmacol Exp Ther. 2019;370(3):682-694.
4. Ciernikova S, Earl J, García Bermejo ML, et al. Epigenetic landscape in pancreatic ductal adenocarcinoma: on the way to overcoming drug resistance? Int J Mol Sci. 2020;21(11):4091.
5. Kato H, Horiguchi A, Ito M, et al. Multimodal treatment of localized pancreatic adenocarcinoma: current topics and updates in survival outcomes and prognostic factors. Ann Gastroenterol Surg. 2021;5:132-151.
6. Khorana AA, Mangu PB, Berlin J, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(21):2541-2556.
7. Khorana AA, McKernin SE, Berlin J, et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:2082-2088.
8. Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(22):2654-2668.
9. Sohal DPS, Mangu PB, Khorana AA, et al. Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(23):2784-2796.
10. Sohal DPS, Kennedy EB, Cinar P, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020;38(27):3217-3230.
11. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(4):439-457.
12. Palta M, Godfrey D, Goodman KA, et al. Radiation therapy for pancreatic cancer: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2019;9(5):322-332.
13. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56-v68.
14. European Society of Medical Oncology. Pancreatic Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Available at https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-gastrointestinal-cancers/cancer-of-the-pancreas-esmo-clinical-practice-guidelines. Last accessed August 25, 2024.
15. National Institute for Health and Care Excellence. Pancreatic Cancer in Adults: Diagnosis and Management. Available at https://www.nice.org.uk/guidance/ng85/resources/pancreatic-cancer-in-adults-diagnosis-and-management-pdf-1837696373701. Last accessed August 25, 2024.
16. Martin-Perez E, Domínguez-Muñoz JE, Botella-Romero F, et al. Multidisciplinary consensus statement on the clinical management of patients with pancreatic cancer. Clin Transl Oncol. 2020;22(11):1963-1975.
17. Iacobuzio-Donahue CA. Genetic evolution of pancreatic cancer: lessons learnt from the pancreatic cancer genome sequencing project. Gut. 2012;61(7):1085-1094.
19. National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. Available at https://seer.cancer.gov/statfacts/html/pancreas.html. Last accessed August 1, 2024.
20. Khoudari G, Alkhayyat M, Abou Saleh M, et al. The epidemiology of pancreatic cancer and the association with acetylsalicylic acid in the United States: a population-based study. Pancreas. 2020;49(9):1207-1212.
21. Tian C, Clauser KR, Öhlund D, et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci U S A. 2019;116(39):19609-19618.
22. Heller DR, Nicolson NG, Ahuja N, et al. Association of treatment inequity and ancestry with pancreatic ductal adenocarcinoma survival. JAMA Surg. 2020;155:e195047.
23. Noel M, Fiscella K. Disparities in pancreatic cancer treatment and outcomes. Health Equity. 2019;3(1):532-540.
24. Dragovich T. Pancreatic Cancer. Available at https://emedicine.medscape.com/article/280605-overview. Last accessed August 25, 2024.
25. Duell EJ, Holly EA, Bracci PM, et al. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. J Natl Cancer Inst. 2002;94(4):297-306.
26. Fernandez-del Castillo C, Jimenez RE. Epidemiology and Nonfamilial Risk Factors for Exocrine Pancreatic Cancer. Available at https://www.uptodate.com/contents/epidemiology-and-nonfamilial-risk-factors-for-exocrine-pancreatic-cancer. Last accessed August 25, 2024.
27. Korc M, Jeon CY, Edderkaoui M, et al. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2017;31(5):529-536.
28. Genkinger JM, Kitahara CM, Bernstein L, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann Oncol. 2015;26:2257-2266.
29. Abbruzzese JL, Andersen DK, Borrebaeck CAK, et al. The interface of pancreatic cancer with diabetes, obesity, and inflammation: research gaps and opportunities. Summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas. 2018;47(5):516-525.
30. Nkondjock A, Ghadirian P, Johnson KC, Krewski D. Dietary intake of lycopene is associated with reduced pancreatic cancer risk.J Nutr. 2005;135(3):592-597.
31. Waterhouse M, Risch HA, Bosetti C, et al. Vitamin D and pancreatic cancer: a pooled analysis from the pancreatic cancer case-control consortium. Ann Oncol. 2015;26(8):1776-1783.
32. Borazanci E, Dang CV, Robey RW, et al. Pancreatic cancer: "a riddle wrapped in a mystery inside an enigma." Clin Cancer Res. 2017;23:1629-1637.
33. Maisonneuve P, Amar S, Lowenfels AB. Periodontal disease, edentulism, and pancreatic cancer: a meta-analysis. Ann Oncol. 2017;28:985-995.
34. Hoare A, Soto C, Rojas-Celis V, Bravo D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediators Inflamm. 2019;2019:1029857.
35. Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328(20):1433-1437
36. Kim NH, Chang Y, Lee SR, et al. Glycemic status, insulin resistance, and risk of pancreatic cancer mortality in individuals with and without diabetes. Am J Gastroenterol. 2020;115(11):1840-1848.
37. Sah RP, Nagpal SJS, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10(7):423-433.
38. Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083.
39. Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928-1937.
40. Grote VA, et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: a study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Diabetologia. 2011;54:3037-3046.
41. Ogawa Y, et al. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002;94:2344-2349.
42. Cho J, Scragg R, Pandol SJ, et al. Antidiabetic medications and mortality risk in individuals with pancreatic cancer-related diabetes and postpancreatitis diabetes: a nationwide cohort study. Diabetes Care. 2019;42:1675-1683.
43. Sadeghi N, Abbruzzese JL, Yeung SCJ, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18(10):2905-2912.
44. Chaiteerakij R, Petersen GM, Bamlet WR, et al. Metformin use and survival of patients with pancreatic cancer: a cautionary lesson.J Clin Oncol. 2016;34:1898-1904.
45. Toriola AT, Stolzenberg-Solomon R, Dalidowitz L, Linehan D, Colditz G. Diabetes and pancreatic cancer survival: a prospective cohort-based study. Br J Cancer. 2014;111(1):181-185.
46. Sah RP, Sharma A, Nagpal S, et al. Phases of metabolic and soft tissue changes in months preceding a diagnosis of pancreatic ductal adenocarcinoma. Gastroenterology. 2019;156(6):1742-1752.
47. Liao WC, Chen PR, Huang CC, et al. Relationship between pancreatic cancer-associated diabetes and cachexia. J Cachexia Sarcopenia Muscle. 2020;11(4):899-908.
48. Felsenstein M, Hruban RH, Wood LD. New developments in the molecular mechanisms of pancreatic tumorigenesis. Adv Anat Pathol. 2018;25:131-142.
49. Hruban RH. Molecular Pathogenesis of Exocrine Pancreatic Cancer. Available at https://www.uptodate.com/contents/molecular-pathogenesis-of-exocrine-pancreatic-cancer. Last accessed August 25, 2024.
50. Mortoglou M, Tabin ZK, Arisan ED, et al. Non-coding RNAs in pancreatic ductal adenocarcinoma: new approaches for better diagnosis and therapy. Transl Oncol. 2021;14(7):101090.
51. Evan GI, Hah N, Littlewood TD, et al. Re-engineering the pancreas tumor microenvironment: a "regenerative program" hacked. Clin Cancer Res. 2017;23(7):1647-1655.
52. Rodríguez Gil Y, Jiménez Sánchez P, Muñoz Velasco R, García García A, Sánchez-Arévalo Lobo VJ. Molecular alterations in pancreatic cancer: transfer to the clinic. Int J Mol Sci. 2021;22(4):2077.
53. Riva G, Pea A, Pilati C, et al. Histo-molecular oncogenesis of pancreatic cancer: from precancerous lesions to invasive ductal adenocarcinoma. World J Gastrointest Oncol. 2018;10(10):317-327.
54. Morani AC, Hanafy AK, Ramani NS, et al. Hereditary and sporadic pancreatic ductal adenocarcinoma: current update on genetics and imaging. Radiol Imaging Cancer. 2020;2(2):e190020.
55. Daly MB, Pal TP, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021. J Natl Compr Canc Netw. 2021;19:77-102.
56. Connor AA, Denroche RE, Jang GH, et al. Integration of genomic and transcriptional features in pancreatic cancer reveals increased cell cycle progression in metastases. Cancer Cell. 2019;35(2):267-282.
57. Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25(5):579-586.
58. Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6(8):2969-2972.
59. Parkin AA, Man J, Chou A, et al. The evolving understanding of the molecular and therapeutic landscape of pancreatic ductal adenocarcinoma. Diseases. 2018;6:103.
60. Hanada K, Amano H, Abe T. Early diagnosis of pancreatic cancer: current trends and concerns. Ann Gastroenterol Surg. 2017; 1:44-51.
61. Stephens PJ, Greenman CD, Fu B, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144(1):27-40.
62. Dreyer SB, Chang DK, Bailey P, Biankin AV. Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin Cancer Res. 2017;23(7):1638-1646.
63. Notta F, Chan-Seng-Yue M, Lemire M, et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature. 2016;538(7625):378-382.
64. Chan-Seng-Yue M, Kim JC, Wilson GW, et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat Genet. 2020;52(2):231-240.
65. Pompella L, Tirino G, Pappalardo A, et al. Pancreatic cancer molecular classifications: from bulk genomics to single cell analysis. Int J Mol Sci. 2020;21(8):2814.
66. Iovanna J. Implementing biological markers as a tool to guide clinical care of patients with pancreatic cancer. Translational Oncology. 2021;14:100965.
67. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185-203.
68. Barcellini A, Peloso A, Pugliese L, et al. Locally advanced pancreatic ductal adenocarcinoma: challenges and progress. Onco Targets and Therapy. 2020;13:12705-12720.
69. Vennin C, Murphy KJ, Morton JP, Cox TR, Pajic M, Timpson P. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology. 2018;154(4):820-838.
70. Canto MI. Familial Risk Factors for Pancreatic Cancer and Screening of High-Risk Patients. Available at https://www.uptodate.com/contents/familial-risk-factors-for-pancreatic-cancer-and-screening-of-high-risk-patients. Last accessed August 25, 2024.
71. Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020;69:7.
72. Earl J, Galindo-Pumariño C, Encinas J, et al. A comprehensive analysis of candidate genes in familial pancreatic cancer families reveals a high frequency of potentially pathogenic germline variants. EBioMedicine. 2020;53:102675.
73. Stoffel EM, McKernin SE, Brand R, et al. Evaluating susceptibility to pancreatic cancer: ASCO provisional clinical opinion. J Clin Oncol. 2019; 37(2):153-164.
74. U.S. Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for pancreatic cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322(5):438-444.
75. McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846-4861.
76. Mills K, Birt L, Emery JD, et al. Understanding symptom appraisal and help-seeking in people with symptoms suggestive of pancreatic cancer: a qualitative study. BMJ Open. 2017;7(9):e015682.
77. Keane MG, Horsfall L, Rait G, Pereira SP. A case-control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open. 2014;4(11):e005720.
78. Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma.J Clin Oncol. 2017;35(30):3382-3390.
79. Mamon H. Initial Chemotherapy and radiation For Nonmetastatic, Locally Advanced, Unresectable and Borderline Resectable, Exocrine Pancreatic Cancer. Available at https://www.uptodate.com/contents/initial-chemotherapy-and-radiation-for-nonmetastatic-locally-advanced-unresectable-and-borderline-resectable-exocrine-pancreatic-cancer. Last accessed August 25, 2024.
80. Ryan DP. Initial Systemic Chemotherapy for Metastatic Exocrine Pancreatic Cancer. Available at https://www.uptodate.com/contents/initial-systemic-chemotherapy-for-metastatic-exocrine-pancreatic-cancer. Last accessed August 25, 2024.
81. Nguyen M. Pancreatic cancer. In: Merck Manual Professional Version. Kenilworth, NJ: Merck Sharp & Dohme Corp; 2021.
82. Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7(5):189-197.
83. Turaga KK, Malafa MP, Jacobsen PB, Schell M, Sarr MG. Suicide in patients with pancreatic cancer. Cancer. 2011;117(3):642-647.
84. Fernandez-del Castillo C. Clinical Manifestations, Diagnosis, and Staging of Exocrine Pancreatic Cancer. Available at https://www.uptodate.com/contents/clinical-manifestations-diagnosis-and-staging-of-exocrine-pancreatic-cancer. Last accessed August 25, 2024.
85. Puckett Y, Garfield K. Pancreatic Cancer. Available at https://www.ncbi.nlm.nih.gov/books/NBK518996. Last accessed July 19, 2024.
86. Kordes M, Larsson L, Engstrand L, Löhr JM. Pancreatic cancer cachexia: three dimensions of a complex syndrome. Br J Cancer. 2021;124(10):1623-1636.
87. Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Gastroenterology. 2014;146:291-304.
88. Tummers WS, Willmann JK, Bonsing BA, Vahrmeijer AL, Gambhir SS, Swijnenburg RJ. Advances in diagnostic and intraoperative molecular imaging of pancreatic cancer. Pancreas. 2018;47(6):675-689.
89. Al-Hawary M. Role of imaging in diagnosing and staging pancreatic cancer. J Natl Compr Canc Netw. 2016;14(5.5):678-680.
90. Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. Journal of Gastrointestinal Oncology. 2012;3:105-120.
91. Giannis D, Moris D, Barbas AS. Diagnostic, predictive and prognostic molecular biomarkers in pancreatic cancer: an overview for clinicians. Cancers. 2021;13:1071.
92. Zhao B, Cheng Q, Cao H, et al. Dynamic change of serum CA19-9 levels in benign and malignant patients with obstructive jaundice after biliary drainage and new correction formulas. BMC Cancer. 2021;21:517.
93. Kakar S, Pawlik TM, Allen PJ, et al. Exocrine pancreas. In: Amin MB (ed). AJCC Cancer Staging Manual. 8th ed. Chicago, IL: American Joint Committee on Cancer; 2017.
94. Chawla A, Wo J, Castillo CF, et al. Clinical staging in pancreatic adenocarcinoma underestimates extent of disease. Pancreatology. 2020;20(4):691-697.
96. Elsayed M, Abdelrahim M. The latest advancement in pancreatic ductal adenocarcinoma therapy: a review article for the latest guidelines and novel therapies. Biomedicines. 2021;9:389.
97. Neeman E, Gresham G, Ovasapians N, et al. Comparing physician and nurse Eastern Cooperative Oncology Group performance status (ECOG-PS) ratings as predictors of clinical outcomes in patients with cancer. Oncologist. 2019;24:e1460-e1466.
98. Mamon H. Treatment for Potentially Resectable Exocrine Pancreatic Cancer. Available at https://www.uptodate.com/contents/treatment-for-potentially-resectable-exocrine-pancreatic-cancer. Last accessed August 25, 2024.
99. National Cancer Institute. Pancreatic Cancer: Health Professional Version. Available at https://www.cancer.gov/types/pancreatic/hp. Last accessed August 13, 2024.
100. Torgeson A, Garrido-Laguna I, Tao R, Cannon GM, Scaife CL, Lloyd S. Value of surgical resection and timing of therapy in patients with pancreatic cancer at high risk for positive margins. ESMO Open. 2018;3(1):e000282.
101. Cellini F, Arcelli A, Simoni N, et al. Basics and frontiers on pancreatic cancer for radiation oncology: target delineation, SBRT, SIB technique, MRgRT, particle therapy, immunotherapy and clinical guidelines. Cancers (Basel). 2020;12(7):1729.
102. Fernandez-del Castillo C, Jimenez RE. Overview of Surgery in the Treatment of Exocrine Pancreatic Cancer and Prognosis. Available at https://www.uptodate.com/contents/overview-of-surgery-in-the-treatment-of-exocrine-pancreatic-cancer-and-prognosis. Last accessed August 25, 2024.
103. Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19(1):169-175.
104. Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10(1):16425.
105. Brenner T, Duggal S, Natale J, Goldberg RM. Treatment Protocols for Pancreatic Cancer. Available at https://www.uptodate.com/contents/treatment-protocols-for-pancreatic-cancer. Last accessed August 25, 2024.
106. Goldberg RM. Second-Line Systemic Therapy for Advanced Exocrine Pancreatic Cancer. Available at https://www.uptodate.com/contents/second-line-systemic-therapy-for-advanced-exocrine-pancreatic-cancer. Last accessed August 25, 2024.
107. The Medical Letter. In brief: pembrolizumab (Keytruda) for cancers with biomarkers. Med Lett Drugs Ther. 2018;60(1537):e8.
108. Golan T, Hammel P. Management of BRCA mutation carriers with pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2021;19(4):469-473.
109. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317-327.
110. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357;(6349):409-413.
111. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481.
112. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267-277.
113. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395-2406.
114. Fromer MW, Hawthorne J, Philips P, et al. An improved staging system for locally advanced pancreatic cancer: a critical need in the multidisciplinary era. Ann Surg Oncol. 2021;28(11):6201-6210.
115. Abrams RA, Winter KA, Safran H, et al. Results of the NRG Oncology/RTOG 0848 adjuvant chemotherapy question: erlotinib+gemcitabine for resected cancer of the pancreatic head: a phase II randomized clinical trial. Am J Clin Oncol. 2020;43(3):173-179.
116. Patel SH, Katz MHG, Ahmad SA. The landmark series: preoperative therapy for pancreatic cancer. Ann Surg Oncol. 2021;28(8):4104-4129.
117. Fong ZV, Ferrone CR. Surgery after response to chemotherapy for locally advanced pancreatic ductal adenocarcinoma: a guide for management. J Natl Compr Canc Netw. 2021;19(4):459-467.
118. Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020-1027.
119. van Roessel S, van Veldhuisen E, Klompmaker S, et al. Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol. 2020;6(11):1733-1740.
120. White RR, Murphy JD, Martin RCG. The landmark series: locally advanced pancreatic cancer and ablative therapy options. Ann Surg Oncol. 2021;28(8):4173-4180.
121. Reyngold M, O'Reilly EM, Varghese AM, et al. Association of ablative radiation therapy with survival among patients with inoperable pancreatic cancer. JAMA Oncol. 2021;7(5):735-738.
122. Fernandez-del Castillo C, Jimenez RE, Murphy JE. Supportive Care of the Patient with Locally Advanced or Metastatic Exocrine Pancreatic Cancer. Available at https://www.uptodate.com/contents/supportive-care-for-locally-advanced-or-metastatic-exocrine-pancreatic-cancer. Last accessed August 25, 2024.
123. Drewes AM, Campbell CM, Ceyhan GO, et al. Pain in pancreatic ductal adenocarcinoma: a multidisciplinary, international guideline for optimized management. Pancreatology. 2018;18:446-457.
124. Powell-Brett S, Pande R, Roberts KJ. Achieving "marginal gains" to optimize outcomes in resectable pancreatic cancer. Cancers. 2021;13:1669.
125. Society on Sarcopenia, Cachexia and Wasting Disorders. Cachexia. Available at https://society-scwd.org/cachexia. Last accessed August 25, 2024.
126. Loprinzi CL, Jatoi A. Management of Cancer Anorexia/Cachexia. Available at https://www.uptodate.com/contents/management-of-cancer-anorexia-cachexia. Last accessed August 25, 2024.
127. Currow DC, Glare P, Louw S, et al. A randomised, double blind, placebo-controlled trial of megestrol acetate or dexamethasone in treating symptomatic anorexia in people with advanced cancer. Sci Rep. 2021;11(1):2421.
128. Hunter CN, Abdel-Aal HH, Elsherief WA, et al. Mirtazapine in cancer-associated anorexia and cachexia: a double-blind placebo-controlled randomized trial. J Pain Symptom Manage. 2021:3924(21):00369.
129. Mücke M, Weier M, Carter C, et al. Systematic review and meta-analysis of cannabinoids in palliative medicine. J Cachexia Sarcopenia Muscle. 2018;9(2):220-234.
130. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS ONE. 2021;16(3):e0246990.
131. Roeland EJ, Bohlke K, Baracos VE, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol. 2020;38(21):2438-2453.
132. Forsmark CE, Tang G, Xu H, et al. The use of pancreatic enzyme replacement therapy in patients with a diagnosis of chronic pancreatitis and pancreatic cancer in the US is infrequent and inconsistent. Aliment Pharmacol Ther. 2020;51(10):958-967.
133. Casolino R, Braconi C, Malleo G, et al. Reshaping preoperative treatment of pancreatic cancer in the era of precision medicine.Ann Oncol. 2021;32(2):183-196.
135. Sung H, Siegel RL, Rosenberg, PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3);e137-e147.
136. Rainone MR, Singh I, Salo-Mullen EE, et al. An emerging paradigm for germline testing in pancreatic ductal adenocarcinoma and immediate implications for clinical practice: a review. JAMA Oncol. 2020;6:764-771.
137. Aslanian HR, Lee JH, Canto MI, et al. AGA clinical practice update on pancreas cancer screening in high-risk individuals: Expert Review. Gastroenterology. 2020;159:358-362.
138. Wang C, Cai H, Cai Q, et al. Circulating microRNAs in association with pancreatic cancer risk within 5 years. Int J Cancer. 2024;155:519-531.
139. DuBois JS, Kambadakone A, Wo JY, et al. Case 19-2022: a 29-year-old woman with jaundice and chronic diarrhea. NEJM. 2022;386:2413-2423.
140. U.S. Food and Drug Administration. FDA approves irinotecan liposome for first-line treatment of metastatic pancreatic adenocarcinoma. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-irinotecan-liposome-first-line-treatment-metastatic-pancreatic-adenocarcinoma. Last accessed August 25, 2024.
141. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology : Pancreatic Adenocarcinoma Version 2.2024. Available at https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Last accessed August 25, 2024.
1. Khorana AA, McKernin SE, Berlin J, et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019;37(23):2082-2088. Available at https://ascopubs.org/doi/10.1200/JCO.19.00946. Last accessed August 27, 2024.
2. Palta M, Godfrey D, Goodman KA, et al. Radiation therapy for pancreatic cancer: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2019;9(5):322-332. Available at https://www.practicalradonc.org/cms/10.1016/j.prro.2019.06.016/attachment/0e8abbe7-fcc6-4c5d-8b46-e81e636ce080/mmc1.pdf. Last accessed August 27, 2024.
3. Sohal DPS, Kennedy EB, Cinar P, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020;38(27):3217-3230. Available at https://ascopubs.org/doi/10.1200/JCO.20.01364. Last accessed August 27, 2024.
Mention of commercial products does not indicate endorsement.